-
Name
Methimazole
- EINECS 200-482-4
- CAS No. 60-56-0
- Article Data31
- CAS DataBase
- Density 1.28 g/cm3
- Solubility soluble in water
- Melting Point 144-147 °C(lit.)
- Formula C4H6N2S
- Boiling Point 154.8 °C at 760 mmHg
- Molecular Weight 114.171
- Flash Point 47.4 °C
- Transport Information
- Appearance White Solid
- Safety 26-27-45-36/37
- Risk Codes 43-62-63
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 4-Imidazoline-2-thione,1-methyl- (6CI,7CI);Imidazole-2-thiol, 1-methyl- (8CI);1-Methyl-1H-imidazole-2-thiol;1-Methyl-2-mercapto-1H-imidazole;1-Methyl-4-imidazoline-2-thione;1-Methylimidazole-2-thiol;1-Methylimidazole-2-thione;Basolan;Danantizol;Mercaptazole;Mercazole;Mercazolyl;
- PSA 56.62000
- LogP 0.70880
Synthetic route
-
-
79487-94-8
2-tert-butylthio-1-methyl-1H-imidazole

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride at 20℃; Reagent/catalyst; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-1H-imidazole With n-butyllithium In tetrahydrofuran; hexane at -10℃; for 1.5h; Stage #2: With sulfur In tetrahydrofuran; hexane for 9h; Temperature; Reflux; | 82.54% |

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol at 20℃; for 5h; Solvent; | 77% |
-
-
63348-55-0
2-(benzylthio)-1-methyl-1H-imidazole

-
A
-
23269-08-1
3-methyl-1-benzyl-1,3-dihydroimidazole-2-thione

-
B
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| In methanol for 5h; Irradiation; | A 168 mg B 88 mg |
-
-
7647-01-0
hydrogenchloride

-
-
333-20-0
potassium thioacyanate

-
-
20677-73-0
N-methylaminoacetaldehyde diethyl acetal

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
90203-43-3
N-(2,2-diethoxy-ethyl)-N'-methyl-thiourea

-
-
60-56-0
2-Mercapto-1-methylimidazole
-
-
3129-90-6
isothiocyanic acid

-
-
20677-73-0
N-methylaminoacetaldehyde diethyl acetal

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With Carbonate buffer at 25℃; Kinetics; |
-
-
333-20-0
potassium thioacyanate

-
-
20677-73-0
N-methylaminoacetaldehyde diethyl acetal

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20 - 30℃; Large scale; | 150 kg |
-
-
540-72-7
sodium thiocyanide

-
-
122-07-6
N-(methyl)aminoacetaldehyde dimethyl acetal

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20 - 60℃; pH=1 - 2; | 0.86 kg |
-
-
33252-29-8
6-chloro-pyridine-2-carbonitrile

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With sodium hydride In DMF (N,N-dimethyl-formamide) at 70℃; for 18h; | 100% |
-
-
99519-64-9
1-bromo-3-(4-chloro-phenyl)-3-methyl-butan-2-one

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
1185752-50-4
3-(4-chloro-phenyl)-3-methyl-1-(1-methyl-1H-imidazol-2-ylsulfanyl)-butan-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 40 - 45℃; for 72h; Darkness; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 40 - 45℃; for 72h; Darkness; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 40 - 45℃; for 72h; Darkness; | 100% |
-
-
42599-17-7
(E)-1-iodo-1-octene

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With potassium phosphate; (1,10-phenanthroline)bis(triphenylphosphine)copper(I) nitrate In toluene at 110℃; for 24h; | 99% |
-
-
15278-97-4
(trimethylphosphine)gold(I) chloride

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
1355252-24-2
(C3H3N2(CH3)S)(trimethylphosphine gold(I))

| Conditions | Yield |
|---|---|
| With NaOH In methanol to thiol-compd. in CH3OH NaOH added, stirred for 5 min at room temp., Au-compd. added, stirred for 1 h; evaporated in vac., elem. anal.; | 99% |
-
-
15529-90-5
(triethylphosphine)chlorogold(I)

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
1355252-25-3
(C3H3N2(CH3)S)(triethylphosphine gold(I))

| Conditions | Yield |
|---|---|
| With NaOH In methanol to thiol-compd. in CH3OH NaOH added, stirred for 5 min at room temp., Au-compd. added, stirred for 1 h; evaporated in vac., elem. anal.; | 99% |
-
-
14243-64-2
(triphenylphosphine)gold(I) chloride

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
1355252-26-4
(C3H3N2(CH3)S)(triphenylphosphine gold(I))

| Conditions | Yield |
|---|---|
| With NaOH In methanol to thiol-compd. in CH3OH NaOH added, stirred for 5 min at room temp., Au-compd. added, stirred for 1 h; evaporated in vac., elem. anal.; | 99% |
-
-
70314-82-8
N-(2,4-dimethylphenyl)-2,2,2-trifluoroethanimidoyl chloride

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-Mercapto-1-methylimidazole With sodium hydride In acetonitrile at 25℃; for 0.5h; Stage #2: N-(2,4-dimethylphenyl)-2,2,2-trifluoroethanimidoyl chloride In acetonitrile at 25℃; for 9h; | 99% |
-
-
69563-07-1
2,2,2-trifluoro-N-(3-(trifluoromethyl)phenyl)acetimidoyl chloride

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-Mercapto-1-methylimidazole With sodium hydride In acetonitrile at 25℃; for 0.5h; Stage #2: 2,2,2-trifluoro-N-(3-(trifluoromethyl)phenyl)acetimidoyl chloride In acetonitrile at 25℃; for 9h; | 99% |
-
-
762-42-5
dimethyl acetylenedicarboxylate

-
-
603-35-0
triphenylphosphine

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 5h; | 98% |

-
-
14447-03-1
Diisopropyl acetylenedicarboxylate

-
-
603-35-0
triphenylphosphine

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 5h; | 98% |


| Conditions | Yield |
|---|---|
| With triphenylphosphine | 98% |
-
-
78056-39-0
4,5-difluoro-2-nitroaniline

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
849236-50-6
5-(1-methyl-1H-imidazol-2-ylthio)-4-fluoro-2-nitrobenzenamine

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 60℃; for 5h; | 98% |
-
-
14242-05-8
silver perchlorate monohydrate

-
-
37095-27-5
(bis(diphenylphosphino)methane)bis(chlorogold(I))

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: AgCl; N2-atmosphere, in dark; addn. of 2 equiv. AgClO4 to Au-complex, standingfor 0.5 h, filtration, addn. of stoich. amt. mercaptoimidazole, standin g for 0.5 h (pptn.); collection (centrifugation); second crop from mother liquor; elem. anal.; | 98% |


| Conditions | Yield |
|---|---|
| In methanol for 3h; | 98% |

-
-
66086-33-7
Di-tert-butyl acetylenedicarboxylate

-
-
603-35-0
triphenylphosphine

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 5h; | 97% |

-
-
16940-66-2
sodium tetrahydroborate

-
-
563-68-8
thallium(I) acetate

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol; water byproducts: CH3COONa; suspn. of NaBH4 and S compd. in THF refluxed for 15 h under Ar, cooled to room temp., solvent removed in vac., solid dissolved in MeOH, aq. soln. of TlOAc added, stirred for 15 min; filtered, washed with water, dried in vac. for 2.5 h; elem. anal.; | 97% |

-
-
4225-92-7
2-bromo-1-(2,4,6-trimethylphenyl)ethanone

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
1154952-09-6
2-(1-methyl-1H-imidazol-2-ylsulfanyl)-1-(2,4,6-trimethyl-phenyl)-ethanone

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; | 97% |
-
-
1185023-08-8
2-bromo-1-[1-(4-chloro-phenyl)-cyclopropyl]-ethanone

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
1185751-91-0
1-[1-(4-chloro-phenyl)-cyclopropyl]-2-(1-methyl-1H-imidazol-2-ylsulfanyl)-ethanone

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| In ethanol for 36h; Reflux; | 97% |
-
-
155262-17-2
2-fluoro-5-phenyl-3-trifluoromethylthiophene

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,4-dioxane Heating; | 96% |
-
-
603-35-0
triphenylphosphine

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
762-21-0
acetylenedicarboxylic acid diethyl ester

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 5h; | 96% |

-
-
658040-87-0
7-methoxy-2-methylsulfonyl-3-phenyl-4H-1-benzopyran-4-one

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; | 96% |
-
-
138286-76-7, 5445-29-4
ethyl-2-bromooctanoate

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
212769-61-4
2-(1-methyl-1H-imidazol-2-yl-sulfanyl)-octanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| 96% | |
| 96% |
-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
137048-30-7
1-methyl-1H-imidazole-2-sulfonic acid

| Conditions | Yield |
|---|---|
| With chlorine dioxide In water for 0.5h; | 96% |
-
-
42599-24-6
E-styryl iodide

-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-Mercapto-1-methylimidazole With potassium fluoride; copper(l) iodide; cis-1,2-cyclohexane In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: E-styryl iodide In N,N-dimethyl-formamide at 90℃; for 12h; | 96% |
-
-
75-09-2
dichloromethane

-
-
60-56-0
2-Mercapto-1-methylimidazole

-
-
133213-39-5
1,1'-methylenebis(1,3-dihydro-3-methyl-1H-imidazole-2-thione)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol at 80℃; for 26h; Sealed tube; | 95.8% |
| at 79.84℃; for 24h; | 89% |
-
-
60-56-0
2-Mercapto-1-methylimidazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 3h; Ambient temperature; | 95% |
Methimazole Chemical Properties
IUPAC Name: 3-Methyl-1H-imidazole-2-thione
Synonyms of Methimazole (CAS NO.60-56-0): 1,3-Dihydro-1-methyl-2H-imidazole-2-thione ; 1-Methyl-1,3-dihydroimidazole-2-thione ; 1-Methyl-2-mercaptoimidazole ; 1-Methylimidazole-2-thiol ; 2-Mercapto-1-methylimidazole ; 4-Imidazoline-2-thione, 1-methyl- ; Favistan ; Mercazolylum ; N-Methyl-2-mercaptoimidazole
CAS NO: 60-56-0
Molecular Formula: C4H6N2S
Molecular Weight: 114.16
Molecular Structure: 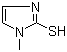
EINECS: 200-482-4
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 0
Polar Surface Area: 38.57 Å2
Index of Refraction: 1.666
Molar Refractivity: 33.07 cm3
Molar Volume: 88.8 cm3
Surface Tension: 61.9 dyne/cm
Density: 1.28 g/cm3
Flash Point: 47.4 °C
Enthalpy of Vaporization: 39.15 kJ/mol
Boiling Point: 154.8 °C at 760 mmHg
Vapour Pressure: 3.13 mmHg at 25°C
Melting point: 140-145 °C
Water solubility: soluble
Appearance: White Solid
SMILES: S=C1N(\C=C/N1)C
InChI: InChI=1/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7)
InChIKey: PMRYVIKBURPHAH-UHFFFAOYAN
Std. InChI: InChI=1S/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7)
Std. InChIKey: PMRYVIKBURPHAH-UHFFFAOYSA-N
SMILES: S=C1N(\C=C/N1)C
InChI: InChI=1/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7)
InChIKey: PMRYVIKBURPHAH-UHFFFAOYAN
Std. InChI: InChI=1S/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7)
Std. InChIKey: PMRYVIKBURPHAH-UHFFFAOYSA-N
Product Categories of Methimazole (CAS NO.60-56-0): Heterocyclic Compounds;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals
Methimazole Uses
Methimazole (CAS NO.60-56-0) is used as a thiourea antithyroid agent that prevents iodine organification, thus inhibiting the synthesis of thyroxine.
Methimazole Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 500mg/kg (500mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | oral | 860mg/kg (860mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR | Arzneimittel-Forschung. Drug Research. Vol. 4, Pg. 20, 1954. |
| mouse | LD50 | subcutaneous | 345mg/kg (345mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR | Arzneimittel-Forschung. Drug Research. Vol. 4, Pg. 20, 1954. |
| rat | LD50 | oral | 2250mg/kg (2250mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR | Arzneimittel-Forschung. Drug Research. Vol. 4, Pg. 20, 1954. |
| rat | LD50 | subcutaneous | 1050mg/kg (1050mg/kg) | Farmaco, Edizione Scientifica. Vol. 14, Pg. 54, 1959. | |
| women | TDLo | oral | 13mg/kg/33D-I (13mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EAR BEHAVIORAL: EXCITEMENT GASTROINTESTINAL: OTHER CHANGES | Clinical Endocrinology Vol. 51, Pg. 667, 1999. |
| women | TDLo | oral | 492mg/kg/88W- (492mg/kg) | ENDOCRINE: CHANGE IN GONADOTROPINS BLOOD: AGRANULOCYTOSIS | Netherlands Journal of Medicine. Vol. 43, Pg. 71, 1993. |
Methimazole Consensus Reports
Reported in EPA TSCA Inventory.
Methimazole Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 43-62-63
43: May cause sensitization by skin contact
62: Possible risk of impaired fertility
63: Possible risk of harm to the unborn child
Safety Statements: 26-27-45-36/37
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
27: Take off immediately all contaminated clothing
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37: Wear suitable protective clothing and gloves
WGK Germany: 3
RTECS: NI8615000
HS Code: 29332990
Poison by subcutaneous route. Moderately toxic by ingestion and intraperitoneal routes. Human teratogenic effects. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental neoplastigenic data. Human mutation data reported. An antithyroid drug. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also MERCAPTANS.
Methimazole Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Avoid ingestion and inhalation. Use only in a chemical fume hood.
Storage: Store in a cool, dry place. Store in a tightly closed container.
Related Products
- Methimazole
- 60560-05-6
- 60560-22-7
- 60561-89-9
- 60562-16-5
- 605624-20-2
- 605-62-9
- 60563-13-5
- 60563-36-2
- 6056-35-5
- 605644-46-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View