-
Name
Methanethiol
- EINECS 200-822-1
- CAS No. 74-93-1
- Article Data374
- CAS DataBase
- Density 0.814 g/cm3
- Solubility
- Melting Point -123 °C(lit.)
- Formula CH4S
- Boiling Point 6 °C(lit.)
- Molecular Weight 48.1088
- Flash Point < 71 °C
- Transport Information UN 2037 2.3
- Appearance colourless gas with a garlic-like or rotten cabbage-like smell
- Safety 16-25-60-61
- Risk Codes 12-23-50/53
-
Molecular Structure
-
Hazard Symbols
 F+,
F+, T,
T, N
N
- Synonyms Mercaptomethane;Thiomethanol;Methanethiol;Methyl sulfhydrate;Thiomethyl alcohol;Mercaptan methylique;
- PSA 38.80000
- LogP 0.54600
Synthetic route
-
-
39076-43-2
S-methyl N-ethylthiocarbamate

-
-
74-89-5
methylamine

-
A
-
74-93-1
methylthiol

-
B
-
28145-10-0
1-ethyl-3-methylurea

| Conditions | Yield |
|---|---|
| In water at 50 - 55℃; for 1h; | A n/a B 100% |

-
-
75-04-7
ethylamine

-
-
39076-43-2
S-methyl N-ethylthiocarbamate

-
A
-
74-93-1
methylthiol

-
B
-
623-76-7
N,N'-diethylurea

| Conditions | Yield |
|---|---|
| In water at 40℃; for 8h; | A n/a B 100% |

-
-
10191-60-3
dimethyl N-cyanodithioiminocarbonate

-
-
100-51-6
benzyl alcohol

-
A
-
74-93-1
methylthiol

-
B
-
50909-46-1
benzyloxycarbonylcyanamide potassium salt

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) 80-90 deg C, 48 h; 2.) 25-30 deg C 24 h; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| With hydrogen sulfide at 260 - 800℃; under 6000.6 Torr; for 0.216667h; Temperature; Pressure; Inert atmosphere; Calcination; | 98.4% |
| With hydrosulfide anion In gas Rate constant; Thermodynamic data; reaction efficiency, ΔH0Rx; | |
| With sodium hydrogensulfide; water unter Druck; |
-
-
98773-05-8, 115958-66-2
trichlorogallium * ethanethiol

-
-
3908-55-2
Trimethyl(methylthio)silane

-
A
-
75-77-4
chloro-trimethyl-silane

-
D
-
74-93-1
methylthiol

-
E
-
75-08-1
ethanethiol

| Conditions | Yield |
|---|---|
| In benzene 4.62 mmol Me3SiSMe added to 4.62 mmol of the Ga compound in benzene; stirring at room temp.; filtration; solvent removed in vac.; elem. anal. (Cl2GaSC2H5); | A n/a B 98% C n/a D n/a E n/a |

-
-
98773-05-8, 115958-66-2
trichlorogallium * ethanethiol

-
-
35029-96-0
lead bis(methylthiolate)

-
C
-
74-93-1
methylthiol

-
D
-
75-08-1
ethanethiol

| Conditions | Yield |
|---|---|
| In benzene 2.10 mmol Pb(SCH3)2 added to 4.20 mmol of the Ga compound in benzene; stirring at room temp.; filtration; solvent removed in vac.; residue washed with n-pentane and dried in vac.; elem. anal. (Cl2GaSC2H5); | A 98% B n/a C n/a D n/a E n/a |

-
-
5863-81-0, 112528-10-6, 112528-11-7
methyl N-benzyl-N′-cyanocarbamimidothioate

-
A
-
74-93-1
methylthiol

-
B
-
21505-06-6
N3-benzyl-1H-1,2,4-triazole-3,5-diamine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A n/a B 97% |
-
-
166829-91-0
1-cyan-3-(3'-amino-3',5'-dideoxythymidin-5'-yl)-2-methylisothiourea

-
A
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With silver nitrate In N,N-dimethyl-formamide for 18h; Ambient temperature; protected from light; | A n/a B 96% |

| Conditions | Yield |
|---|---|
| With ammonia In water at 65℃; | A n/a B 96% |
-
-
32885-06-6
S-methyl-N''-cyano-N'-(1-cycloethoxyethyl)carbamimidothioate

-
A
-
51420-46-3
5-amino-3-morpholino-1H-1,2,4-triazole

-
B
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A 95% B n/a |
-
-
37067-45-1
N1,S-Dimethylthiobenzohydrazoniumiodid

-
A
-
74-93-1
methylthiol

-
B
-
83274-32-2
N2-Methylbenzamidrazoniumiodid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol for 24h; Ambient temperature; | A n/a B 95% |
-
-
22013-97-4
N-methyl-thiocarbamic acid S-methyl ester

-
-
111-92-2
dibutylamine

-
A
-
74-93-1
methylthiol

-
B
-
21260-54-8
N,N-dibutyl-N'-methyl urea

| Conditions | Yield |
|---|---|
| at 65 - 70℃; | A n/a B 95% |


| Conditions | Yield |
|---|---|
| With hydrogen sulfide; catalyst according to WO 2005/021491; catalyst according to PCT/EP/2005/012898 with 9.5wtpercent Br content at 320 - 380℃; under 6750.68 Torr; Product distribution / selectivity; Gas phase; | 94.5% |
| With hydrogen sulfide; zinc(II) chloride at 260 - 390℃; under 3750.38 - 7500.75 Torr; Temperature; Pressure; | 93.7% |
| With hydrogen sulfide; catalyst according to PCT/EP/2005/012898 with 9.5wtpercent Br content at 320 - 370℃; under 6750.68 Torr; Product distribution / selectivity; Gas phase; | 92% |
-
-
1122-62-9
2-acetylpyridine

-
-
20184-94-5
N-methyl-hydrazinecarbodithioic acid methyl ester

-
A
-
74-93-1
methylthiol

-
B
-
74752-59-3
methyl (E,Z,E)-<4-(3,4-dihydro-2,6-dimethyl-3-thioxo-1,2,4-triazin-5(2H)-ylidene)-2-butenylidene>methylhydrazinecarbodithioate

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 4h; Heating; | A n/a B 93% |

-
-
65159-19-5
1-Aza-3-thia-1-cyan-2-piperidino-1-buten

-
A
-
74-93-1
methylthiol

-
B
-
51420-45-2
5-amino-2-(piperidinyl)-1H-1,2,4-triazole

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A n/a B 93% |
-
-
21504-96-1
methyl N-phenyl-N′-cyanocarbamimidothioate

-
A
-
3310-68-7
N3-phenyl-1H-1,2,4-triazole-3,5-diamine

-
B
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A 93% B n/a |
-
-
106580-89-6
methyl N'-cyano-N-[(pyridin-3-yl)methyl]-imidothiocarbamate

-
A
-
74-93-1
methylthiol

-
B
-
106580-72-7
5-amino-3-(pyridin-3-ylmethylamino)-1H-1,2,4-triazole

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A n/a B 92% |
-
-
12107-04-9
dicarbonyl(cyclopentadienyl)iron(II) chloride

-
-
10543-35-8
lithium methylsulfinyl carbanion

-
A
-
38117-54-3
cyclopentadienyl iron(II) dicarbonyl dimer

-
B
-
74-93-1
methylthiol

-
C
-
624-92-0
Dimethyldisulphide

-
D
-
6628-18-8
1,2-bis(methylthio)ethane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran mixing reactants in THF at -78°C, slow warming to room temp.; evapn. in vac., extn. with pentane, ether and finally acetone or CH2Cl2, concn., chromy. on Al2O3, purifn. by crystn., distn. or sublimation; | A 55% B n/a C 21% D 8% E 92% |

-
-
105448-13-3
7-Chlor-1-methyl-2-methylthio-5-phenyl-1H-1,3,4-benzotriazepin

-
A
-
74-93-1
methylthiol

-
B
-
1022-13-5
5-chloro-2-(methylamino)benzophenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 10h; Heating; | A n/a B 91% |
-
-
72499-72-0
C10H11N3S

-
A
-
16691-44-4
N3-o-tolyl-1H-[1,2,4]triazole-3,5-diyldiamine

-
B
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A 91% B n/a |
-
-
59541-69-4
1-cyano-2-methyl-3-p-tolyl-isothiourea

-
A
-
3929-18-8
N3-(p-tolyl)-1H-1,2,4-triazole-3,5-diamine

-
B
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A 91% B n/a |
-
-
132628-97-8
1-methylsulfanyl-3,3-dimethyl-3,4-dihydroisoquinoline

-
-
107-95-9
3-amino propanoic acid

-
A
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| In ethanol for 4h; Substitution; Heating; | A n/a B 91% |

-
-
37067-45-1
N1,S-Dimethylthiobenzohydrazoniumiodid

-
A
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With ammonia In dichloromethane | A n/a B 90% |
-
-
15048-19-8
Cyanoguanyl-imido-dithiokohlensaeuredimethylester

-
-
141-43-5
ethanolamine

-
A
-
74-93-1
methylthiol

-
B
-
128937-36-0
C5H9N5O2

| Conditions | Yield |
|---|---|
| In ethanol Heating; | A n/a B 89.5% |

-
-
106-44-5
p-cresol

-
-
6216-98-4
S-Methyl-O-p-tolyl-thiocarbonate

-
A
-
74-93-1
methylthiol

-
B
-
621-02-3
di-p-tolyl carbonate

| Conditions | Yield |
|---|---|
| With sodium carbonate In decane for 1h; Heating; | A n/a B 89% |

-
-
106580-90-9
C9H10N4S

-
A
-
74-93-1
methylthiol

-
B
-
106580-73-8
5-amino-3-(pyridine-4-ylmethylamino)-1H-1,2,4-triazole

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Heating; | A n/a B 89% |
-
-
63-68-3
L-methionine

-
-
132628-97-8
1-methylsulfanyl-3,3-dimethyl-3,4-dihydroisoquinoline

-
A
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| In ethanol for 16h; Substitution; Heating; | A n/a B 89% |

-
-
1271-19-8
bis(cyclopentadienyl)titanium dichloride

-
-
10543-35-8
lithium methylsulfinyl carbanion

-
B
-
74-93-1
methylthiol

-
C
-
624-92-0
Dimethyldisulphide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran mixing reactants in THF at -78°C, slow warming to room temp.; evapn. in vac., extn. with pentane, ether and finally acetone or CH2Cl2, concn., chromy. on Al2O3, purifn. by crystn., distn. or sublimation; | A 31% B n/a C 9% D 43% E 89% |

-
-
73491-54-0
N-Nitro-amidino-dithiocarbamidsaeuredimethylester

-
-
137-07-5
2-amino-benzenethiol

-
A
-
74-93-1
methylthiol

-
B
-
113513-25-0
C8H7N5O2S

| Conditions | Yield |
|---|---|
| In ethanol Heating; | A n/a B 88.4% |

-
-
5856-63-3
(2R)-2-aminobutan-1-ol

-
-
126232-74-4
Acetic acid 1-cyano-2,2-bis-methylsulfanyl-vinyl ester

-
A
-
74-93-1
methylthiol

-
B
-
126232-68-6
methyl (R)-(-)-(E)-(4-ethyl-2-oxazolidinylidene)cyanoacetate

| Conditions | Yield |
|---|---|
| In ethanol for 10h; Heating; | A n/a B 88% |

-
-
674-82-8
4-methyleneoxetan-2-one

-
-
74-93-1
methylthiol

-
-
108615-97-0
4-Methylsulfanylmethyl-oxetan-2-one

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In diethyl ether for 2h; Ambient temperature; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) at -20℃; for 6h; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) at -20℃; for 3h; Irradiation; | 100% |
| With 2,2'-azobis(isobutyronitrile) at -20℃; for 3h; Irradiation; Yield given; |
-
-
74-93-1
methylthiol

-
-
13654-95-0
isobutyl ethenesulfonate

-
-
120263-37-8
isobutyl 2-methylthioethanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 15h; Ambient temperature; | 100% |
-
-
74-93-1
methylthiol

-
-
14109-62-7
ethyl 2-acetamido-2-(ethoxycarbonyl)-4-pentenoate

-
-
125773-33-3
diethyl 2-acetamido-2-<3-(methylthio)propyl>malonate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In ethanol at 50℃; for 66h; | 100% |
-
-
74-93-1
methylthiol

-
-
37944-29-9
ethyl 2-acetamido-2-ethoxycarbonyl-4-methyl-4-pentenoic acid

-
-
144072-84-4
diethyl 2-acetamido-2-<2-methyl-3-(methylthio)propyl>malonate

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In ethanol at 50℃; for 60h; | 100% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide In N,N,N,N,N,N-hexamethylphosphoric triamide at 20℃; | 100% |
-
-
74-93-1
methylthiol

-
-
136463-55-3, 140241-92-5
(2aR*9aR*) 2a,9-dimethyl-1-phenyl-1,2a,3,4,9,9a-hexahydro-2H-azeto <2,3-b> <1> benzoazepin-2-one

-
-
136463-63-3, 136463-64-4
N-phenyl (2R*3S*) 1,3-dimethyl-2-methylthio-2,3,4,5-tetrahydro-1H-1-benzoazepine-3-carboxamide

| Conditions | Yield |
|---|---|
| In dichloromethane at -50℃; for 5h; | 100% |
-
-
74-93-1
methylthiol

-
-
144125-67-7
Cα-methyl-D,L-allylglycinamide

-
-
144073-00-7
2-amino-2-methyl-5-(methylthio)pentanoic acid amide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In methanol at 60℃; for 24h; | 100% |
-
-
74-93-1
methylthiol

-
-
107640-15-3
3-(2-Bromethyl)-5-trifluormethyl-1,3,4-oxadiazol-2(3H)-on

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 3h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane; acetic acid for 0.00138889h; | 100% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone 1.) CH2Cl2, AcOH, r.t.; 2.) CH2Cl2; Yield given. Multistep reaction; |
-
-
5460-29-7
2-(3-bromopropyl)isoindole-1,3-dione

-
-
74-93-1
methylthiol

-
-
52096-79-4
2-<2-(Methylthio)propyl>-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol | 100% |

| Conditions | Yield |
|---|---|
| In chloroform for 5h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at -75 - 25℃; for 5h; | 100% |
-
-
74-93-1
methylthiol

-
-
65213-15-2
sp-9-(o-tert-butylphenyl)-9-fluorenol

-
-
226384-22-1
ap-9-(o-tert-butylphenyl)-9-methylthiofluorene

| Conditions | Yield |
|---|---|
| With hydrogen bromide at -15℃; Condensation; | 100% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methanesulfonic acid In water at 40 - 110℃; for 10h; | 100% |
| With dihydrogen peroxide In water at 35 - 45℃; for 10h; | 100% |
| With nitric acid at 80℃; for 8h; Concentration; Temperature; | 93.75% |
| With Mg(2+)*O4V(3-)*C19H42N(1+); dihydrogen peroxide In acetonitrile at 20 - 40℃; for 25h; Reagent/catalyst; Temperature; | 63.9% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone | 100% |
-
-
74-93-1
methylthiol

-
-
650-52-2
bis(trifluoromethyl)chlorophosphine

-
-
1486-18-6
Bis--methyl-thio-phosphin

| Conditions | Yield |
|---|---|
| byproducts: HCl; in presence of HCl initializer; | 100% |
| byproducts: HCl; in presence of HCl initializer; | 97% |
| byproducts: HCl; in presence of HCl initializer; | 97% |

| Conditions | Yield |
|---|---|
| In benzene under N2, CH3SH introduced in a suspension of AlCl3 for 5 min, dissoln.of AlCl3, stirred for 15 min at room temp.; evapn. of solvent, dried in vac. at room temp. for 3h, elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In benzene under N2, CH3SH introduced in a suspension of AlBr3 for 7 min, dissoln.of AlBr3, stirred for 15 min at room temp.; evapn. of solvent, dried in vac. at room temp. for 3h, elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: CH4; (under N2); the thiol is introduced into a soln. of MeGaI2 in benzene; the solvent is evapd., the residue is washed, dried; elem. anal.; | 100% |
-
-
94-59-7
1-allyl-3,4-methylenedioxybenzene

-
-
74-93-1
methylthiol

-
-
5304-80-3
5-(3-(methylthio)propyl)benzo[d][1,3]dioxole

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Glovebox; Sealed tube; | 100% |
-
-
74-93-1
methylthiol

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Inert atmosphere; Glovebox; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Inert atmosphere; Glovebox; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Inert atmosphere; Glovebox; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Inert atmosphere; Glovebox; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Inert atmosphere; Glovebox; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With C38H28Au2F12FeN2O8P2S4 In 1,4-dioxane at 45℃; for 20h; Inert atmosphere; Glovebox; Sealed tube; | 100% |
Methyl mercaptan Consensus Reports
Methyl mercaptan Standards and Recommendations
DFG MAK: 0.5 ppm (1 mg/m3)
NIOSH REL: (n-Alkane Monothiols) CL 0.5 ppm/15M
DOT Classification: 2.3; Label: Poison Gas, Flammable Gas
Methyl mercaptan Analytical Methods
Methyl mercaptan Specification
The Methyl mercaptan, with the CAS registry number 74-93-1, is also known as Thiomethanol. It belongs to the product category of API intermediates. Its EINECS number is 200-822-1. This chemical's molecular formula is CH4S and molecular weight is 48.11. What's more, its systematic name is methanethiol. Its classification codes are: (1)Mutation data; (2)TSCA Flag T [Subject to the Section 4 test rule under TSCA]. It is an intermediate in the manufacturing of jet fuels, pesticides, fungicides, plastics, synthesis of methionine. Moreover, its odor may cause nausea. It is also used in the production of intermediates of methane sulfonyl chloride and methylthio propanol. It should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides and fire.
Physical properties of Methyl mercaptan are: (1)ACD/LogP: 0.72; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.72; (4)ACD/LogD (pH 7.4): 0.72; (5)ACD/BCF (pH 5.5): 2.06; (6)ACD/BCF (pH 7.4): 2.06; (7)ACD/KOC (pH 5.5): 58.46; (8)ACD/KOC (pH 7.4): 58.42; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 25.3 Å2; (13)Index of Refraction: 1.408; (14)Molar Refractivity: 14.57 cm3; (15)Molar Volume: 59 cm3; (16)Polarizability: 5.77×10-24cm3; (17)Surface Tension: 19.2 dyne/cm; (18)Density: 0.814 g/cm3; (19)Enthalpy of Vaporization: 23.79 kJ/mol.
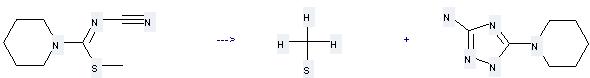
Preparation: this chemical can be prepared by 1-Aza-3-thia-1-cyan-2-piperidino-1-buten by heating. This reaction will need reagent 72% N2H4·H2O and solvent ethanol. The yield is about 93%.
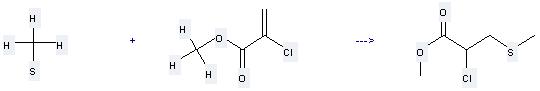
Uses of Methyl mercaptan: it can be used to produce 2-chloro-3-methylsulfanyl-propionic acid methyl ester at the ambient temperature. It will need reagent NaSCH3 and solvent CH2Cl2 with the reaction time of 4 hours. The yield is about 87%.
When you are using this chemical, please be cautious about it as the following:
This chemical is extremely flammable, so you should keep it away from sources of ignition - No smoking. It is toxic by inhalation and is very toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. When using it, you must avoid contact with eyes. This material and its container must be disposed of as hazardous waste. You must avoid releasing it to the environment just refering to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: SC
(2)Std. InChI: InChI=1S/CH4S/c1-2/h2H,1H3
(3)Std. InChIKey: LSDPWZHWYPCBBB-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | unreported | 60670ug/kg (60.67mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 16(6), Pg. 46, 1972. | |
| mouse | LC50 | inhalation | 6530ug/m3/2H (6.53mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 16(6), Pg. 46, 1972. | |
| rat | LC50 | inhalation | 675ppm (675ppm) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED | Personal Communication from H.B. Lackey, Chemical Products Div., Crown Zellerbach, Camas, WA 98607, June 9, 1978Vol. 09JUN1978. |
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 7493-57-4
- 7493-58-5
- 7493-63-2
- 74936-72-4
- 7493-69-8
- 7493-72-3
- 7493-74-5
- 7493-76-7
- 74938-90-2
- 7493-95-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View