-
Name
Methyl pyruvate
- EINECS 209-987-4
- CAS No. 600-22-6
- Article Data72
- CAS DataBase
- Density 1.072 g/cm3
- Solubility Soluble in chloroform, methanol, ether, alcohol. Slightly soluble in water.
- Melting Point -22 °C
- Formula C4H6O3
- Boiling Point 135.5 °C at 760 mmHg
- Molecular Weight 102.09
- Flash Point 39.4 °C
- Transport Information UN 3272 3/PG 3
- Appearance Clear colourless to yellow liquid
- Safety 16
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms Pyruvicacid, methyl ester (6CI,7CI,8CI);2-Oxo-propionic acid methyl ester;2-Oxopropanoic acid methyl ester;Methyl 2-oxopropanoate;Methyl2-oxopropionate;Methyl acetoformate;Methyl pyroracemate;Methyl pyruvate;Methylglyoxylic acid methyl ester;NSC 65430;Pyroracemic acid methyl ester;
- PSA 43.37000
- LogP -0.25160
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc(II) nitrate; hydroxylamine hydrochloride; oxygen; copper(II) nitrate; sodium hydroxide at 100℃; under 75.0075 Torr; for 8h; Molecular sieve; Autoclave; Green chemistry; | 98.9% |
| With p-methoxybenzenetellurinic acid anhydride In neat (no solvent) at 130℃; for 1h; | 90% |
| With hydrogenchloride; sodium hypobromide In dichloromethane; water at 25℃; for 5h; | 90% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 83℃; Temperature; | 76.38% |
| With ethanesulfonic acid; 1,2-dichloro-ethane |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid at 24.84℃; under 760.051 Torr; Kinetics; Mechanism; UV-irradiation; | A n/a B 76% |
| With ozone at 20.84℃; under 760 Torr; Kinetics; Darkness; | A 55% B 69% |
-
-
480-96-6
benzofurazan oxide

-
-
2605-68-7
methyl 2-(triphenylphosphoranylidene)propionate

-
A
-
273-09-6
benzofurazan

-
B
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| In benzene for 6h; Heating; | A 45% B n/a |


| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; triethylamine; chromium(VI) oxide In acetonitrile at 40℃; for 20h; Product distribution; variation of catalyst, reagent, temperature, time; | 41% |
| With dihydrogen peroxide; triethylamine; chromium(VI) oxide In acetonitrile at 40℃; for 20h; | 41% |
| Stage #1: methacrylic acid methyl ester With cis-[Ru(2,9-Me2phen)2(OH2)2](PF6)2 In acetonitrile at 55℃; for 0.5h; Stage #2: With dihydrogen peroxide In acetonitrile for 8h; |

| Conditions | Yield |
|---|---|
| With carbon dioxide; 1,3-bis[2,6-di(propan-2-yl)phenyl]-2-methoxyimidazolidine In tetrahydrofuran at 20℃; for 8h; Inert atmosphere; Sealed tube; | 10% |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide |
-
-
35356-70-8
Methyl 2-acetamidoacrylate

-
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogen bromide In chloroform |
-
-
91765-79-6
1,4-Bis-methoxycarbonyl-1,2,3,4-tetramethyl-butadien-1,3

-
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| (i) O3, MeOH, (ii) H2, Pd-C; Multistep reaction; |
-
-
67-56-1
methanol

-
-
1809-02-5
2-chloro-3-methylbutadiene

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
109-87-5
Dimethoxymethane

-
C
-
79-20-9
acetic acid methyl ester

-
D
-
10076-48-9
methyl 2,2-dimethoxypropionate

-
E
-
80-62-6
methacrylic acid methyl ester

-
F
-
108365-85-1
2-Chlor-3,3-dimethoxy-1-buten

| Conditions | Yield |
|---|---|
| With ozone at -78℃; Product distribution; mono- and diozonolysies; with and without work up with DMS; | A n/a B n/a C 35 % Spectr. D 47 % Spectr. E 7 % Spectr. F 93 % Spectr. |

-
-
67-56-1
methanol

-
-
108365-81-7
(2-Chlor-1-methoxy-1-methyl-2-propenyl)hydroperoxide

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
109-87-5
Dimethoxymethane

-
C
-
79-20-9
acetic acid methyl ester

-
D
-
10027-72-2
methoxymethyl hydroperoxide

-
E
-
64-19-7
acetic acid

-
F
-
108365-82-8
methyl α-hydroperoxy-α-methoxypropionate

| Conditions | Yield |
|---|---|
| With ozone In 1,1,2,2-tetrachloroethylene at -50℃; Product distribution; ozonolysis; |


| Conditions | Yield |
|---|---|
| With 4-(dimethylamino)pyridine N-oxide In acetonitrile for 0.333333h; Heating; | 90 % Chromat. |
-
-
56410-68-5
3-chloro-DL-alanine methyl ester

-
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| With H2O In water at 25℃; Rate constant; Thermodynamic data; Kinetics; E(act.), ΔH(act.), ΔS(act.); with or without pyridoxal methochloride; |

| Conditions | Yield |
|---|---|
| With perchloric acid at 25℃; Rate constant; |
-
-
96204-70-5
C10H14O10

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
547-64-8
methyl lactate

-
C
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| In benzene at 55℃; Rate constant; Product distribution; oth. temperature. oth. solvent; |

-
-
105949-86-8
3-carbomethoxy-3-methyl-1,2,4-trioxolane

-
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| With dimethylsulfide In chloroform-d1 Yield given; |

| Conditions | Yield |
|---|---|
| With (η5-C5H4SiMe3)2NbH(O)C=CPh2 at 25℃; |
-
-
67-56-1
methanol

-
-
80-62-6
methacrylic acid methyl ester

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
4461-52-3
methoxymethanol

-
C
-
10027-72-2
methoxymethyl hydroperoxide

-
D
-
105949-86-8
3-carbomethoxy-3-methyl-1,2,4-trioxolane

-
E
-
108365-82-8
methyl α-hydroperoxy-α-methoxypropionate

| Conditions | Yield |
|---|---|
| With ozone at -78℃; Product distribution; ozonolysis; |

-
-
328-42-7
Oxalacetic acid

-
-
74-88-4
methyl iodide

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
63921-06-2
3-methyl-2-oxo-butane-1,4-dioic acid dimethyl ester

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |

-
-
67-56-1
methanol

-
-
127-17-3
2-oxo-propionic acid

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
10076-48-9
methyl 2,2-dimethoxypropionate

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; 2,2-dimethoxy-propane for 24h; Ambient temperature; | A 71 % Chromat. B 29 % Chromat. |


| Conditions | Yield |
|---|---|
| With hydroxide at 23.85℃; under 760 Torr; Kinetics; Oxidation; |
-
-
53358-15-9
cis-form of γ-methyl-glutaconic acid dimethyl ester

-
-
10028-15-6
ozone

-
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| in der Waerme am Licht; |
-
-
554-12-1
propanoic acid methyl ester

-
A
-
600-22-6
pyruvic acid methyl ester

-
B
-
922-68-9
Methyl glyoxylate

-
C
-
10500-31-9
Propionic formic anhydride

-
D
-
802294-64-0
propionic acid

| Conditions | Yield |
|---|---|
| With air; Propanil at 22.85℃; under 740 Torr; Kinetics; Oxidation; | A 0.289 mol B 0.111 mol C 0.099 mol D 0.139 mol |


| Conditions | Yield |
|---|---|
| With hydroxylamine In 1,4-dioxane; water at 30℃; pH=6.75; Equilibrium constant; |
-
-
80-62-6
methacrylic acid methyl ester

-
A
-
58653-97-7
methyl 2-methyl-2-oxiranecarboxylate

-
B
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| With Nitrogen dioxide; ozone at 250℃; for 4.44444E-06h; gas phase; | A 88 % Spectr. B n/a |

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; polymer-bound NADH (2a) In acetonitrile; benzene at 80℃; for 120h; Further byproducts given; | 100% |
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II); hydrogen; sodium hydrogencarbonate In methanol at 25℃; under 7500.75 Torr; for 12h; Glovebox; Autoclave; chemoselective reaction; | 98% |
| With hydrogen; Et4N | 91% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
63584-41-8
1-ethylthio-1-(trimethylsiloxy)ethene

-
-
127658-10-0
(S)-ethyl 3-hydroxy-3-methoxycarbonylbutanethioate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; (S,S)-Cu(II) complex from bis(oxazolinyl) ligand and Cu(OTf)2 In tetrahydrofuran at -78℃; | 100% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
17392-83-5
(R)-Methyl lactate

| Conditions | Yield |
|---|---|
| With hydrogen; Cinchonidin; polyvinylpyrrolidone-stabilized platinum clusters In acetic acid at 25℃; under 30002.4 Torr; for 0.5h; | 100% |
| With hydrogen; butan-1-ol; PVP-Pt; Cinchonidin In acetic acid at 24.85℃; under 30002.4 Torr; for 0.5h; | |
| Stage #1: bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; (R)-N-diphenylphosphino-N-methyl-[(S)-2-(diphenylphosphino)ferrocenyl]ethylamine In tetrahydrofuran at 25℃; for 0.25h; Stage #2: pyruvic acid methyl ester With hydrogen In tetrahydrofuran under 1277.21 - 1794.37 Torr; for 6h; Product distribution / selectivity; | n/a |
-
-
600-22-6
pyruvic acid methyl ester

-
-
507989-55-1
4,4-bis(benzyloxycarbonyl)-1,7-dimethylhepta-1,6-diyne

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In dichloromethane at 20℃; for 0.166667h; | 100% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
187607-97-2
methyl pyruvate 2-chloro-5-methylphenylhydrazone

| Conditions | Yield |
|---|---|
| In benzene | 100% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
27871-49-4
(S)-Methyl lactate

| Conditions | Yield |
|---|---|
| With hydrogen; Cinchonin In acetic acid at 25℃; under 7500.75 Torr; for 12h; enantioselective reaction; | 99.8% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
41978-94-3
2-methoxy-4-nitrophenylhydrazine

| Conditions | Yield |
|---|---|
| With sodium acetate In methanol at 20℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With (S)-[1,1']-binaphthalenyl-2,2'-diol; titanium(IV) isopropylate In diethyl ether; toluene at -20℃; for 60h; Friedel-Crafts reaction; | 99% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
519168-62-8
2-(1-allyl-2-oxoindolin-3-ylidene)malononitrile

-
-
1370462-66-0
(R)-methyl 1-allyl-2'-amino-3'-cyano-2-oxospiro[indoline-3,4'-pyran]-6'-carboxylate

| Conditions | Yield |
|---|---|
| With 1-(((1R,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl)methyl)-3-((1R)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)-methyl)thiourea In diethyl ether at 20℃; for 0.5h; asymmetric Michael cyclization; Molecular sieve; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
1370462-98-8
2-(2-oxo-1-phenylindolin-3-ylidene)malononitrile

-
-
1370462-64-8
(R)-methyl 2'-amino-3'-cyano-2-oxo-1-phenylspiro[indoline-3,4'-pyran]-6'-carboxylate

| Conditions | Yield |
|---|---|
| With 1-(((1R,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl)methyl)-3-((1R)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)-methyl)thiourea In diethyl ether at 20℃; for 3h; asymmetric Michael cyclization; Molecular sieve; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
6623-89-8
2-oxo-2,3-dihydroindolylidenemalononitrile

-
-
1370462-61-5
(R)-methyl 2'-amino-3'-cyano-2-oxospiro[indoline-3,4'-pyran]-6'-carboxylate

| Conditions | Yield |
|---|---|
| With 1-(((1R,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl)methyl)-3-((1R)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)-methyl)thiourea In diethyl ether at 20℃; for 12h; asymmetric Michael cyclization; Molecular sieve; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
7521-41-7
2-Aminonicotinaldehyde

-
-
600-22-6
pyruvic acid methyl ester

-
-
215523-34-5
1,8-naphthyridine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 16h; Cooling; | 99% |
| With water; sodium hydroxide In ethanol at 0 - 20℃; for 18h; |
-
-
600-22-6
pyruvic acid methyl ester

-
-
145259-91-2
Benzoic acid (2S,3R,4R,4aS,10aR)-3-acetylamino-2-benzyloxy-6,6,8,8-tetraisopropyl-hexahydro-1,5,7,9-tetraoxa-6,8-disila-benzocycloocten-4-yl ester

-
-
143836-21-9
Benzyl 2-acetamido-3-O-benzoyl-2-deoxy-4,6-O-<1-(methoxycarbonyl)ethylidene>-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane for 15h; Ambient temperature; | 98% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
62-53-3
aniline

-
-
26458-36-6
methyl 2-methyl-5-oxo-1-phenyl-4-(phenylamino)-2,5-dihydro-1H-pyrrole-2-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 25℃; for 1h; Irradiation; | 98% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
55991-65-6
[1-(4-methoxyphenyl)vinyloxy]trimethylsilane

-
-
1010691-58-3
(R)-methyl 2-hydroxy-2-methyl-4-oxo-4-(4-methoxy-phenyl)butanoate

| Conditions | Yield |
|---|---|
| With (S)-N-[2-[(4S,5R)-4-methyl-5-phenyl-4,5-dihydro-2-oxazolyl]phenyl]-S-methyl-S-phenylsulfoximine; copper(II) bis(trifluoromethanesulfonate) In 2,2,2-trifluoroethanol; toluene at -20℃; for 16h; Mukaiyama reaction; optical yield given as %ee; enantioselective reaction; | 98% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
106-49-0
p-toluidine

-
-
1333149-53-3
dimethyl 2,6‐dimethyl‐1,2‐dihydroquinoline‐2,4‐dicarboxylate

| Conditions | Yield |
|---|---|
| With TiO2 nanoparticles In neat (no solvent) at 80℃; for 1h; Green chemistry; | 98% |
| With iodine In acetonitrile at 50℃; for 12h; | 94% |
| With N-chloro-succinimide; O,O-bis(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl) hydrazine-1,2-bis(carbothioate) In acetonitrile at 60℃; Green chemistry; | 92% |
| With lithium carbonate; magnesium bromide In neat (no solvent) at 60℃; for 1h; regioselective reaction; | 78% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
149-73-5
trimethyl orthoformate

-
-
10076-48-9
methyl 2,2-dimethoxypropionate

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol for 5h; Heating; | 97% |
| With sulfuric acid In methanol for 4h; Reflux; | 77% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
106-49-0
p-toluidine

-
-
26458-39-9
2-methyl-4-(4-methyl-anilino)-5-oxo-1-p-tolyl-2,5-dihydro-pyrrole-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 25℃; for 1h; Irradiation; | 97% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
106-47-8
4-chloro-aniline

-
-
141214-22-4
methyl 3-(4-chloroanilino)-1-(4-chlorophenyl)-5-methyl-2-oxo-3-pyrroline-5-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 25℃; for 1h; Irradiation; | 97% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
188120-44-7
(4aR,5R,6S)-6-tert-butyldimethylsilyloxy-4,4a,5,6,7,8-hexahydro-4a,5-dimethyl-2(3H)-naphthalenone

-
-
7646-85-7
zinc(II) chloride

-
-
4039-32-1
lithium hexamethyldisilazane

-
-
194789-70-3
(4R,6R,7R,8S)-8-t-Butyldimethylsilyloxy-4-(1'-hydroxy-1'-methoxycarbonyl)ethyl-6,7-dimethylbicyclo[4,4,0]deca-1-en-3-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane | 97% |

-
-
600-22-6
pyruvic acid methyl ester

-
-
61550-02-5
(furan-2-yloxy)-trimethylsilane

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroethanol; (R)-N-[2-(2,4,6-trimethylbenzyl)aminophenyl]-S-(4-biphenyl)-S-methylsulfoximine; copper(II) bis(trifluoromethanesulfonate) In diethyl ether at 20℃; Vinylogous Mukaiyama-type aldol reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 97% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
62-53-3
aniline

-
-
120453-92-1
dimethyl 2‐methyl‐1,2‐dihydroquinoline‐2,4‐dicarboxylate

| Conditions | Yield |
|---|---|
| With iodine In acetonitrile at 50℃; for 13h; | 97% |
| With TiO2 nanoparticles In neat (no solvent) at 80℃; for 1.5h; Green chemistry; | 97% |
| With nitric acid In water; acetonitrile at 80℃; Inert atmosphere; | 95% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
873-74-5
4-Aminobenzonitrile

-
-
1403657-23-7
dimethyl 6-cyano-2-methyl-1,2-dihydroquinoline-2,4-dicarboxylate

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 100℃; under 7500.75 Torr; for 10h; Temperature; Time; Microwave irradiation; | 97% |
| With lithium carbonate; magnesium bromide In neat (no solvent) at 90℃; for 5h; regioselective reaction; | 94% |
-
-
600-22-6
pyruvic acid methyl ester

| Conditions | Yield |
|---|---|
| With carbazic acid In neat (no solvent) at 70℃; for 0.5h; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: C15H30O2Si With lithium hexamethyldisilazane In tetrahydrofuran at -78 - -45℃; for 2h; Inert atmosphere; Stage #2: pyruvic acid methyl ester In tetrahydrofuran at -45℃; for 1h; Inert atmosphere; | 97% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
106046-48-4
methyl 2-methyl-2-(dimethylphenylsilyl)-3-butenoate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane for 3h; from -78 deg C to RT; | 96% |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 20℃; for 1h; | 96% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
76943-94-7
(Z)-1-(tert-butylthio)-1-trimethylsilyloxyprop-1-ene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; (S,S)-Cu(II) complex from bis(oxazolinyl) ligand and Cu(OTf)2 In dichloromethane at -78℃; | 96% |
-
-
600-22-6
pyruvic acid methyl ester

-
-
31469-15-5
1-methoxy-2-methyl-1-trimethylsiloxy-1-propene

| Conditions | Yield |
|---|---|
| [bis(diphenylphosphino)methane]bis(propenyl)Ru(II) In tetrahydrofuran at 65℃; for 20h; Condensation; aldol addition; | 96% |
| Stage #1: pyruvic acid methyl ester; 1-methoxy-2-methyl-1-trimethylsiloxy-1-propene With pentafluorophenylammonium trifluoromethanesulfonimide In toluene at -50 - -45℃; for 1h; Mukaiyama reaction; Inert atmosphere; Stage #2: With hydrogenchloride In methanol; water at 20 - 25℃; for 1h; Mukaiyama reaction; Inert atmosphere; | 68% |
Methyl pyruvate Specification
The Propanoic acid, 2-oxo-,methyl ester, with the CAS registry number 600-22-6, is also known as 2-Oxopropanoic acid methyl ester. It belongs to the product category of Pyruvic Acid Series. Its EINECS number is 209-987-4. This chemical's molecular formula is C4H6O3 and molecular weight is 102.09. What's more, its systematic name is methyl 2-oxopropanoate. It can be used in organic synthesis. It should be sealed and stored in a cool and dry place.
Physical properties of Propanoic acid, 2-oxo-,methyl ester are: (1)ACD/LogP: -0.48; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.48; (4)ACD/LogD (pH 7.4): -0.48; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 13.01; (8)ACD/KOC (pH 7.4): 13.01; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 43.37 Å2; (13)Index of Refraction: 1.386; (14)Molar Refractivity: 22.38 cm3; (15)Molar Volume: 95.1 cm3; (16)Polarizability: 8.87×10-24cm3; (17)Surface Tension: 29.1 dyne/cm; (18)Density: 1.072 g/cm3; (19)Flash Point: 39.4 °C; (20)Enthalpy of Vaporization: 37.29 kJ/mol; (21)Boiling Point: 135.5 °C at 760 mmHg; (22)Vapour Pressure: 7.7 mmHg at 25°C.
Preparation: this chemical can be prepared by lactic acid methyl ester at the temperature of 25 °C. This reaction will need reagents NaOBr, HCl and solvents CH2Cl2, H2O with the reaction time of 5 hours. The yield is about 90%.

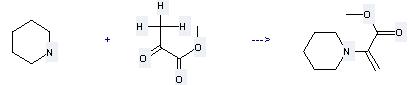
Uses of Propanoic acid, 2-oxo-,methyl ester: it can be used to produce 2-piperidin-1-yl-acrylic acid methyl ester. It will need reagent AsCl3 and solvent diethyl ether with the reaction time of 10 min. The yield is about 73%.
When you are using this chemical, please be cautious about it as the following:
It is flammable, so you should keep it away from sources of ignition - No smoking. You must not breathe vapour. When using it, you need avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(C(=O)OC)C
(2)Std. InChI: InChI=1S/C4H6O3/c1-3(5)4(6)7-2/h1-2H3
(3)Std. InChIKey: CWKLZLBVOJRSOM-UHFFFAOYSA-N
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 60022-62-0
- 60023-35-0
- 6002-40-0
- 60025-09-4
- 60-02-6
- 6003-05-0
- 600-30-6
- 60031-08-5
- 60032-57-7
- 60032-63-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View