-
Name
Methylaminoformyl chloride
- EINECS 229-253-7
- CAS No. 6452-47-7
- Article Data11
- CAS DataBase
- Density 1.185 g/cm3
- Solubility
- Melting Point 45 °C
- Formula C2H4ClNO
- Boiling Point 93oC (dec.)
- Molecular Weight 93.5129
- Flash Point
- Transport Information
- Appearance white crystals
- Safety
- Risk Codes 22
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms Carbamoylchloride, methyl- (7CI);Methylcarbamoyl chloride;N-Methylcarbamoyl chloride;NSC 133003;
- PSA 29.10000
- LogP 0.95550
Synthetic route

| Conditions | Yield |
|---|---|
| In chloroform | 96.7% |


| Conditions | Yield |
|---|---|
| With Phenyltrichlorosilane at 100 - 150℃; for 1h; | A 77.3% B n/a |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane at 15℃; for 0.5h; | 54.4% |
| With hydrogenchloride In tetrachloromethane | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| at 250 - 300℃; | |
| at 250 - 300℃; |

| Conditions | Yield |
|---|---|
| at 275℃; |
-
-
22013-97-4
N-methyl-thiocarbamic acid S-methyl ester

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With chlorine In Dimethyldisulphide at -15 - -10℃; for 0.833333h; |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 25℃; for 16h; | 100% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 25℃; for 16h; Inert atmosphere; | 100% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; | 98% |
-
-
6452-47-7
methylcarbamoyl chloride

-
-
25366-22-7
N-methyl-5-(trifluoromethyl)-1 3,4-thiadiazol-2-amine

-
-
25366-23-8
1-methyl-3-methyl-3-(5-trifluoromethyl-1,3,4-thiadiazol-2-yl)urea

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 30 - 75℃; for 5h; Temperature; Reagent/catalyst; | 97.5% |
-
-
15366-34-4
methyl 1H-pyrazole 3-carboxylate

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -78 - 20℃; for 18h; | 96% |
-
-
1160952-27-1
N-(7-hydroxy-8-methoxy-2-oxo-2H-chromen-3-yl)-3',6-dimethoxy-[1,1'-biphenyl]-3-carboxamide

-
-
6452-47-7
methylcarbamoyl chloride

-
-
1160952-33-9
3-(3',6-dimethoxy-[1,1'-biphenyl]-3-ylcarboxamido)-8-methoxy-2-oxo-2H-chromen-7-yl methylcarbamate

| Conditions | Yield |
|---|---|
| In pyridine for 12h; | 90% |
-
-
850727-84-3
4-amino-1-(3-bromophenyl)-1H-pyrazole-3-carboxamide

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 89% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 5h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 89% |
-
-
5656-45-1
2-hydroxy-S-allylthiophenol

-
-
6452-47-7
methylcarbamoyl chloride

-
-
948-85-6
2-(allylthio)phenyl-N-methylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-S-allylthiophenol With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.5h; Stage #2: methylcarbamoyl chloride In tetrahydrofuran at 0 - 20℃; for 24.25h; | 88% |
-
-
433701-34-9
ethyl 2-amino-4-(3-nitrophenyl)thiophene-3-carboxylate

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; | 87.5% |

-
-
6452-47-7
methylcarbamoyl chloride

-
-
1128193-61-2
N-methyl-3-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl carbamate

| Conditions | Yield |
|---|---|
| Stage #1: 3-(1-benzyl-1H‑1,2,3‑triazol-4-yl)phenol With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.5h; Stage #2: methylcarbamoyl chloride In tetrahydrofuran; water | 86% |

| Conditions | Yield |
|---|---|
| In Isopropylbenzene | 85% |
-
-
1197-34-8
3,5-diethylphenol

-
-
6452-47-7
methylcarbamoyl chloride

-
-
30087-47-9
3,5-diethylphenyl-N-methylcarbamate

| Conditions | Yield |
|---|---|
| In o-xylene | 85% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 16h; | 85% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane Inert atmosphere; | 85% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane at 120℃; for 16h; | 84% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 84% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 25℃; for 8h; | 83% |
| With triethylamine In dichloromethane at 25℃; for 8h; | 83% |

-
-
6452-47-7
methylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 83% |
Methylaminoformyl chloride Specification
The CAS register number of Carbamic chloride,methyl- (9CI) is 6452-47-7. It also can be called as Methylaminoformyl chloride and the IUPAC name about this chemical is N-methylcarbamoyl chloride. The molecular formula about this chemical is C2H4ClNO and the molecular weight is 93.51.
Physical properties about Carbamic chloride,methyl- (9CI) are: (1)ACD/LogP: 0.28; (2)ACD/LogD (pH 5.5): 0.28; (3)ACD/LogD (pH 7.4): 0.28; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 33.76; (7)ACD/KOC (pH 7.4): 33.74; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)Polar Surface Area: 20.31Å2; (11)Index of Refraction: 1.415; (12)Molar Refractivity: 19.78 cm3; (13)Molar Volume: 78.8 cm3; (14)Polarizability: 7.84x10-24cm3; (15)Surface Tension: 29.1 dyne/cm.
Preparation: this chemical can be prepared by isocyanatomethane. This reaction will need reagent HCl.

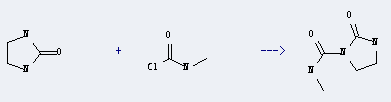
Uses of Carbamic chloride,methyl- (9CI): it can be used to produce 2-oxo-imidazolidine-1-carboxylic acid methylamide with imidazolidin-2-one. The yield is about 38%.
You can still convert the following datas into molecular structure:
(1)SMILES: ClC(=O)NC
(2)InChI: InChI=1/C2H4ClNO/c1-4-2(3)5/h1H3,(H,4,5)
(3)InChIKey: GRRYSIXDUIAUGY-UHFFFAOYAR
(4)Std. InChI: InChI=1S/C2H4ClNO/c1-4-2(3)5/h1H3,(H,4,5)
(5)Std. InChIKey: GRRYSIXDUIAUGY-UHFFFAOYSA-N
Related Products
- Methylaminoformyl chloride
- 64524-77-2
- 64525-01-5
- 6452-57-9
- 6452-61-5
- 6452-71-7
- 64527-29-3
- 64529-56-2
- 64531-21-1
- 64531-49-3
- 64532-22-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View