-
Name
1-FORMAMIDO-1-CYCLOHEXENE
- EINECS
- CAS No. 40652-40-2
- Article Data7
- CAS DataBase
- Density 1.01g/cm3
- Solubility
- Melting Point 58-63oC
- Formula C7H11 N O
- Boiling Point 282.3°C at 760 mmHg
- Molecular Weight 125.17
- Flash Point 158.8°C
- Transport Information
- Appearance
- Safety
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols

- Synonyms N-(1-Cyclohexenyl)formamide
- PSA 29.10000
- LogP 2.21710
Synthetic route
-
-
100-64-1
Cyclohexanone oxime

-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| With 1H-imidazole; titanium acetate; formyl acetic anhydride In N,N-dimethyl-formamide at 25℃; for 4h; | 97% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine; methyloxirane In dichloromethane for 18h; Ambient temperature; | 76% |
-
-
108-94-1
cyclohexanone

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene for 12h; Condensation; Heating; Dean-Stark-conditions; | 76% |
| With sulfuric acid In toluene | 70% |
-
-
1125-02-6
1-cyano-1-formamidocyclohexane

-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 6h; Heating; | 74% |
| With potassium tert-butylate In tetrahydrofuran for 6h; Reflux; Inert atmosphere; | 74% |
| at 590℃; under 14 Torr; |
-
-
108-94-1
cyclohexanone

-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 61 percent / aq. NH4Cl / diethyl ether / 23 °C 2: 53 percent / Ac2O / 12 h / 23 °C 3: 74 percent / t-BuOK / tetrahydrofuran / 6 h / Heating View Scheme |
-
-
5496-10-6
1-amino-1-cyanocyclohexane

-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 53 percent / Ac2O / 12 h / 23 °C 2: 74 percent / t-BuOK / tetrahydrofuran / 6 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: HCO2H 2: 590 °C / 14 Torr View Scheme |
-
-
931-97-5
1-hydroxy-1-cyclohexanecarbonitrile

-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: liq. NH3 / methanol 2: HCO2H 3: 590 °C / 14 Torr View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
1121-57-9
1-isocyanocyclohexene

| Conditions | Yield |
|---|---|
| With pyridine; 1,3,5-trichloro-2,4,6-triazine In dichloromethane at 100℃; for 0.166667h; microwave irradiation; | 96% |
| With benzene-1,3-disulfonyl chloride; triethylamine In dichloromethane at 110℃; for 1h; | 94% |
| With Burgess Reagent In dichloromethane for 12h; Ambient temperature; | 77% |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
A
-
1121-57-9
1-isocyanocyclohexene

-
B
-
128798-30-1
1-Isocyano-7-oxa-bicyclo[4.1.0]heptane

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride; i-PrNEt; 3,3-dimethyldioxirane 1.) CH2Cl2, -40 deg C; 2.) -78 deg C; Yield given. Multistep reaction. Yields of byproduct given; | |
| With trifluoromethylsulfonic anhydride; 3,3-dimethyldioxirane; N-ethyl-N,N-diisopropylamine 1.) CH2Cl2, -40 deg C; 2.) -78 deg C; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
255736-71-1
rac-2-[(2-tert-butyloxycarbonyl-aminoethyl)-(2-thymin-methyl-benzoyl)-amino]-3,3-dimethyl-butyric acid-(cyclohexen-1-yl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / phosgene; NEt3 / CH2Cl2 / 1 h / 0 °C 2: 52 percent / methanol / 48 h / 20 °C View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
255735-94-5
rac-2-[(2-isobutyryl-aminophenyl)-thyminacetyl-amino]-biphenyl-4-yl-acetic acid-(cyclohexen-1-yl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / phosgene; NEt3 / CH2Cl2 / 1 h / 0 °C 2: 51 percent / methanol / 48 h / 20 °C View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / phosgene; NEt3 / CH2Cl2 / 1 h / 0 °C 2: 39 percent / methanol / 48 h / 20 °C View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / phosgene; NEt3 / CH2Cl2 / 1 h / 0 °C 2: 31 percent / methanol / 48 h / 20 °C View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
255735-96-7
rac-2-[(2-tert-butyloxycarbonyl-aminoethyl)-N6-benzyloxycarbonyl-adeninacetyl-amino]-ortho-chlorophenylacetic acid-(cyclohexen-1-yl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / phosgene; NEt3 / CH2Cl2 / 1 h / 0 °C 2: 78 percent / methanol / 48 h / 20 °C View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
171070-14-7
2-[Acetyl-(4-methoxy-benzyl)-amino]-N-cyclohex-1-enyl-3-methyl-butyramide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
171070-16-9
(R,S)-N-(1-cylohexenyl)-2-(N'-(4-methoxybenzyl)formamido)phenylacetamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: 72 percent / methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
171070-15-8
2-[Acetyl-(4-methoxy-benzyl)-amino]-N-cyclohex-1-enyl-2-phenyl-acetamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
171070-17-0
1-[Acetyl-(4-methoxy-benzyl)-amino]-cyclohexanecarboxylic acid cyclohex-1-enylamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
175606-30-1
N-Cyclohex-1-enyl-2-phenyl-2-piperidin-1-yl-acetamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
175606-32-3
N-Butyl-N-[(cyclohex-1-enylcarbamoyl)-phenyl-methyl]-benzamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
175606-21-0
(R,S)-N-(1-cyclohexenyl)-2-(N'-(4-methoxybenzyl)-2-aminobenzamido)-3-methylbutanamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: 1.) 4 A molecular sieves / 1.) CH3OH, 23 deg C, 1 h, 2.) CH3OH, hexane, 23 deg C, 18 h View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
175606-23-2
2-Amino-N-butyl-N-[(cyclohex-1-enylcarbamoyl)-phenyl-methyl]-5-iodo-benzamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: 1.) 4 A molecular sieves / 1.) CH3OH, 23 deg C, 1 h, 2.) CH3OH, hexane, 23 deg C, 18 h View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / diazabicyclo<2.2.2>octane, triethylenediamine, triphosgene / CH2Cl2 / 0.5 h / 0 °C 2: 85 percent / methanol / 12 h / Ambient temperature View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
1354014-33-7
C27H35N3O4

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine; trichlorophosphate / tetrahydrofuran / 0.75 h / 0 °C / Inert atmosphere 2.1: methanol / 0.25 h / 20 °C / Inert atmosphere 2.2: 0.5 h / 20 °C / Inert atmosphere View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

-
-
1426550-76-6
1-cyclohexenyl 3,4,5,7-tetra-O-benzyl-2,6-dideoxy-2,6-(pent-4-enimido)-D-glycero-D-idoheptonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine; trichlorophosphate / dichloromethane / 1 h / -30 °C / Inert atmosphere 2: methanol / 0 - 5 °C / Inert atmosphere View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Burgess Reagent / acetonitrile / 0.17 h / 50 °C / Microwave irradiation 2: ammonia / 2,2,2-trifluoroethanol / 0.17 h / 80 °C / Microwave irradiation View Scheme |
-
-
40652-40-2
N-(cyclohexen-1-yl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Burgess Reagent / acetonitrile / 0.17 h / 50 °C / Microwave irradiation 2: ammonia / 2,2,2-trifluoroethanol / 0.17 h / 80 °C / Microwave irradiation View Scheme |
N-(1-Cyclohexenyl)formamide Chemical Properties
Molecular Structure of N-(1-Cyclohexenyl)formamide (CAS No.40652-40-2):
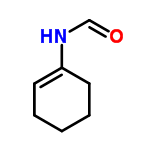
Molecular Formula: C7H11NO
Molecular Weight: 125.17
Systematic Name: N-Cyclohex-1-en-1-ylformamide
CAS No: 40652-40-2
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 1
Polar Surface Area: 20.31 Å2
Index of Refraction: 1.494
Molar Refractivity: 36.08 cm3
Molar Volume: 123.8 cm3
Surface Tension: 35.2 dyne/cm
Density: 1.01 g/cm3
Flash Point: 158.8 °C
Melting point: 58-63 ºC
Enthalpy of Vaporization: 52.12 kJ/mol
Boiling Point: 282.3 °C at 760 mmHg
Vapour Pressure: 0.00338 mmHg at 25°C
InChI: InChI=1/C7H11NO/c9-6-8-7-4-2-1-3-5-7/h4,6H,1-3,5H2,(H,8,9)
InChIKey: PMOWTTQUPBFWRL-UHFFFAOYAJ
Std. InChI: InChI=1S/C7H11NO/c9-6-8-7-4-2-1-3-5-7/h4,6H,1-3,5H2,(H,8,9)
Std. InChIKey: PMOWTTQUPBFWRL-UHFFFAOYSA-N
N-(1-Cyclohexenyl)formamide Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
F: 10-23
N-(1-Cyclohexenyl)formamide Specification
N-(1-Cyclohexenyl)formamide (CAS No.40652-40-2), it also can be called 1-Formamido-1-cyclohexene .
Related Products
- N-(1-Cyclohexenyl)formamide
- 40653-09-6
- 40654-49-7
- 4065-45-6
- 40655-37-6
- 4065-80-9
- 406-58-6
- 4066-02-8
- 40663-68-1
- 4066-39-1
- 4066-41-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View