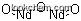-
Name
Neodymium oxide
- EINECS 215-214-1
- CAS No. 1313-97-9
- Density 7.24 g/mL at 20 °C(lit.)
- Solubility insoluble in water
- Melting Point 2270 °C
- Formula Nd2O3
- Boiling Point 3760℃[at 101 325 Pa]
- Molecular Weight 336.48
- Flash Point
- Transport Information
- Appearance blue powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Dineodymiumtrioxide;Neodymia;Neodymium sesquioxide;Neodymium trioxide;Neodymium(3+) oxide;
- PSA 43.37000
- LogP -0.30600
Neodymium oxide Consensus Reports
Neodymium oxide Specification
The Neodymium oxide, with the cas registry number 1313-97-9, has the IUPAC name of neodymium(3+); oxygen(2-). This is a kind of blue powder and is insoluble in water while soluble in aicd. For being a kind of irritant which may cause inflammation to the skin or other mucous, you should avoid contacting with skin and eyes.
Its product categories are including Inorganics; Rare earth; Catalysis and Inorganic Chemistry; Chemical Synthesis; Neodymium; NeodymiumMetal and Ceramic Science; Oxides; 60: Nd; NeodymiumNanomaterials; Materials Science; Nanomaterials; Nanoparticles: Oxides, Nitrides, and Other CeramicsChemical Synthesis; Nanopowders and Nanoparticle Dispersions.
The physical properties of this chemical are as below: (1)#H bond acceptors: 1; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 0; (4)Polar Surface Area: 3; (5)Exact Mass: 333.802546; (6)MonoIsotopic Mass: 331.800182; (7)Topological Polar Surface Area: 3; (8)Heavy Atom Count: 5; (9)Covalently-Bonded Unit Count: 5.
The production method of this chemical is below: Prepare the material of rare earth chloride solvent and then extract with P204-kerosene-HCl-RCl3 system, and divide into three groups of light-weight, middle-weight and heavy-weight; Next get the sediment with oxalic acid and then go through a process of seperation, drying, burning to get this chemical. The chemical equation is below: Nd2(C2O4)3→Nd2O3+3CO2+3CO.
As to its usage, it is widely applied in many fields. It could be used as the colorant agent of glass and ceramics, and the raw material of metal neodymium and NdFeB(neodymium iron boron); It could also be used in the laser technique and also in the preparation of refined instruments.
In addition, you could obtain the molecular structure by using the following datas:
(1)Canonical SMILES: [O-2].[O-2].[O-2].[Nd+3].[Nd+3]
(2)InChI: InChI=1S/2Nd.3O/q2*+3;3*-2
(3)InChIKey: PLDDOISOJJCEMH-UHFFFAOYSA-N
Below are the toxicity informatin of this chemical:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 12, Pg. 625, 1993. |
Related Products
- Neodymium
- Neodymium Acetate
- Neodymium arsenide(NdAs)
- Neodymium bromide
- Neodymium hydroxide
- Neodymium nitride
- Neodymium oxide
- Neodymium trifluoroacetylacetonate
- Neodymium(III) chloride
- Neodymium(III) chloride hexahydrate
- 13139-86-1
- 1313-99-1
- 13140-47-1
- 1314-06-3
- 1314-08-5
- 131410-48-5
- 1314-11-0
- 1314-13-2
- 1314-15-4
- 131417-48-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View