-
Name
Perchloryl fluoride
- EINECS 231-526-0
- CAS No. 7616-94-6
- Article Data34
- CAS DataBase
- Density 1.434
- Solubility miscible
- Melting Point -146℃
- Formula ClFO3
- Boiling Point -46.8℃
- Molecular Weight 102.45
- Flash Point
- Transport Information
- Appearance
- Safety A poison gas which forms methemoglobin in the body and destroys red cells causing anemia, anorexia, and cyanosis. Recovery is said to be rapid, leaving no permanent physiological damage. Can be absorbed through the skin. Its odor can be detected as low as 10 ppm although this cannot be relied upon as an indication of toxic concentration in air. While non-flammable, it supports combustion. It is a powerful oxidizer. Moderately explosive. Potentially explosive reactions with combustible gases or vapors. benzene + aluminum trichloride, benzocyclobutene + butyllithium + potassium tert-butoxide, calcium acetylide, potassium cyanide, potassium thiocyanate, sodium iodide, charcoal, ethyl-4-fluorobenzoylacetate, hydrocarbons, hydrogen sulfide, nitrogen oxide, sulfur dichloride, vinylidene chloride, 3α-hydroxy-5β-androstane-11,17-dione-17-hydrazone, lithiated compounds, 2-lithio(dimethylamino-methyl)ferroxene, methyl-2-bromo-5,5-ethylene dioxy(2,2,1)bicycloheptane-7-carboxylate, aliphatic heterocyclic amines, sodium methoxide + methanol, vinylidene chloride. Reacts to form explosive products with nitrogenous bases (e.g., isopropylamine, isobutylamine, aniline, phenyl hydrazine, 1,2-diphenyl hydrazine), sawdust, lampblack. Violent reaction with finely divided organic materials. A fluorinating agent in chemical synthesis, and an oxidant in rocket fuel. When heated to decomposition it emits toxic fumes of F− and Cl−. See also FLUORINE and PERCHLORATES.
- Risk Codes
-
Molecular Structure
- Hazard Symbols Dangerous in contact with organic materials, strong oxidizing agent. Toxic by inhalation. TLV: 3 ppm; STEL 6 ppm.
- Synonyms Chlorine oxyfluoride;TRIOXYCHLOROFLUORIDE;perchlorylfluoride;Chloryl (per-)fluoride;Chlorine fluoride oxide (ClO3F);EINECS 231-526-0;Perchloryl fluoride ((ClO3)F);Chlorine fluoride oxide;UN3083;
- PSA 51.21000
- LogP 0.75330
Synthetic route

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating at 105°C;; | 97% |
| In neat (no solvent) heating at 105°C;; | 97% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) below -20°C;; passing ClO3F over KI; cooling at -183°C; distillation at -183 - 130°C in vacuum;; | 60% |
| In neat (no solvent) below -20°C;; passing ClO3F over KI; cooling at -183°C; distillation at -183 - 130°C in vacuum;; | 60% |
-
-
3811-04-9
potassium chlorate

-
-
7782-41-4
fluorine

-
A
-
7616-94-6
perchloryl fluoride

-
C
-
10049-04-4, 25052-55-5
chlorine dioxide

-
D
-
7790-89-8
chlorine monofluoride

-
E
-
7782-50-5
chlorine

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: O2; transport reaction: passing F2-N2 mixture over KClO3 at 25 - 60°C;; washing with NaOH; passing ClO3F through Na2S2O3 soln.; drying over NaOH and P2O5;; | A 45% B n/a C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| In water reaction of 60 - 62% aq. HClO4 with SbF5 at 90 - 100°C;; | 36% |
| In water reaction of 60 - 62% aq. HClO4 with SbF5 at 90 - 100°C;; | 36% |
| In neat (no solvent) reaction of 1 mol perchlorate and 3 - 10 mol SbF5 at 100 - 120°C; cooling down;; washing with Na2S2O3; condenstion at temp. of liquid air;; | 36-53 |
-
-
7601-90-3
perchloric acid

-
-
7782-41-4
fluorine

-
A
-
7783-41-7
difluoroether

-
B
-
7616-94-6
perchloryl fluoride

| Conditions | Yield |
|---|---|
| In water |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20 - 40°C;; | 0% |
| In neat (no solvent) at 20 - 40°C;; | 0% |
-
-
7664-39-3
hydrogen fluoride

-
A
-
7783-41-7
difluoroether

-
B
-
7616-94-6
perchloryl fluoride

-
C
-
1333-74-0
hydrogen

-
D
-
80937-33-3
oxygen

-
E
-
7782-50-5
chlorine

| Conditions | Yield |
|---|---|
| In neat (no solvent) Electrolysis; electrolysis of NaClO4 soln. in HF at 0 - 30°C;; condensation with liquid air; washing with Na2S2O3 soln.;; | |
| In neat (no solvent) Electrolysis; electrolysis of NaClO4 soln. in HF at 0 - 30°C;; condensation with liquid air; washing with Na2S2O3 soln.;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heating perchlorate with excess of HSO3F;; | >80 |
| In neat (no solvent) heating perchlorate with excess of HSO3F;; | >80 |

| Conditions | Yield |
|---|---|
| With cesium fluoride byproducts: CO2, Cl2; A mixture of LiClO4 and CsF (as HF getter) was treated in a stainless steel cylinder with COF2; heating up to 120°C; | 0% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating perchlorate with excess of HSO3F;; | >80 |
| In neat (no solvent) heating perchlorate with excess of HSO3F;; | >80 |

| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| In neat (no solvent) reaction of 1 mol perchlorate and 3 - 10 mol SbF5 at 100 - 120°C; cooling down;; washing with Na2S2O3; condenstion at temp. of liquid air;; | 36-53 |

| Conditions | Yield |
|---|---|
| In not given | |
| In fluorosulphonic acid mixt. of KClO4 in fluorosulfonic acid stirred under gradual warming up to about 95°C; FClO3 was flushed with moderate N2 flow; purified through column filled with sodium thiosulfate and ascarite; | |
| In neat (no solvent) heating KClO4 and HSO3F (10:100) at 110°C;; | 52-60 |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating perchlorate with excess of HSO3F;; | >80 |
| In neat (no solvent) heating perchlorate with excess of HSO3F;; | >80 |
-
-
13637-83-7
chloryl fluoride

-
-
7616-94-6
perchloryl fluoride

| Conditions | Yield |
|---|---|
| With water In water byproducts: H2; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating KClO3 and 98% HSO3F at 85°C;; passing ClO3F through thiosulfate soln. and over solid KOH; condensation at temp. of liquid air;; | |
| In neat (no solvent) heating KClO3 and 98% HSO3F at 85°C;; passing ClO3F through thiosulfate soln. and over solid KOH; condensation at temp. of liquid air;; |
-
-
12015-53-1
perchloric anhydride

-
-
7782-41-4
fluorine

-
A
-
7616-94-6
perchloryl fluoride

-
B
-
13637-83-7
chloryl fluoride

-
C
-
7790-89-8
chlorine monofluoride

-
D
-
80937-33-3
oxygen

-
E
-
7782-50-5
chlorine

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; thermal decompn. of Cl2O7 in presence of enough amounts of F2 at 100 - 120°C; reaction mechanism described;; |

-
-
12015-53-1
perchloric anhydride

-
-
7782-41-4
fluorine

-
A
-
7616-94-6
perchloryl fluoride

-
B
-
13637-83-7
chloryl fluoride

| Conditions | Yield |
|---|---|
| In neat (no solvent) thermal decompn. in prescence of F2;; |

-
-
12015-53-1
perchloric anhydride

-
-
7782-41-4
fluorine

-
A
-
7616-94-6
perchloryl fluoride

-
B
-
13637-83-7
chloryl fluoride

-
C
-
7790-89-8
chlorine monofluoride

| Conditions | Yield |
|---|---|
| In neat (no solvent) decompn. of Cl2O7 in presence of F2 at 100 - 120°C;; | |
| In neat (no solvent) decompn. of Cl2O7 in presence of F2 at 100 - 120°C;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) formation as intermediate at 100 - 120°C;; | |
| In neat (no solvent) formation as intermediate at 100 - 120°C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) formation of Cl2O6 as by-product;; | |
| In neat (no solvent) formation of Cl2O6 as by-product;; |

-
-
3811-04-9
potassium chlorate

-
-
7782-41-4
fluorine

-
A
-
7616-94-6
perchloryl fluoride

-
B
-
13637-83-7
chloryl fluoride

-
C
-
7790-89-8
chlorine monofluoride

-
D
-
7782-50-5
chlorine

| Conditions | Yield |
|---|---|
| In neat (no solvent) at -40°C;; | |
| In neat (no solvent) at -40 or 100°C;; fractionated distillation;; | |
| In neat (no solvent) at -40°C;; | |
| In neat (no solvent) at -40 or 100°C;; fractionated distillation;; |

-
-
7616-94-6
perchloryl fluoride

-
-
14268-23-6
potassium trinitromethanide

-
-
1840-42-2
fluorotrinitromethane

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 77% |
| In N,N-dimethyl-formamide | 77% |
| In acetone | 77% |

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 77% |
| In N,N-dimethyl-formamide | 77% |
| In acetone | 77% |
| In methanol at 20°C 11 h; | 17.7% |
-
-
7616-94-6
perchloryl fluoride

-
-
19836-32-9
trinitromethane; ammonium salt

-
-
1840-42-2
fluorotrinitromethane

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 77% |
| In N,N-dimethyl-formamide | 77% |
| In acetone | 77% |
-
-
7616-94-6
perchloryl fluoride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: dimethylphenylamine, N-(4-chloriohenyl)-N-methyl-formamide; dimethyl-(4-trimethylstannanylphenyl)amine dissolved in dry THF was cooled at -60°C; FClO3/N2 mixt. passed through soln. for about 35 min; soln. warmed up to ambient temp. under stirring and stirred at about 60°C for 20 min (closed ....; isolated by semi-preparative thin-layer chromy. (silica gel 60, petroleum ether/ethyl acetate 10:1); | 52% |

| Conditions | Yield |
|---|---|
| In methanol at 20°C 10 h; | 47% |
-
-
7616-94-6
perchloryl fluoride

-
-
940-00-1
(4-methoxyphenyl)trimethylstannane

-
A
-
61726-36-1
bis(4-methoxyphenyl)dimethylstannane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: methoxybenzene; (4-methoxyphenyl)trimethylstannane dissolved in dry THF was cooled at -60°C; FClO3/N2 mixt. passed through soln. for about 35 min; soln. warmed up to ambient temp. under stirring and stirred at about 60°C for 20 min (closed conditions) or; isolated by semi-preparative thin-layer chromy. (silica gel 60, petroleum ether/ethyl acetate 10:1); | A n/a B 40% |

-
-
7616-94-6
perchloryl fluoride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: phenylcarbamic acid tert-butyl ester; (4-trimethylstannanylphenyl)carbamic acid tert-butyl ester dissolved in dry THF was cooled at -60°C; FClO3/N2 mixt. passed through soln. for about 35 min; soln. warmed up to ambient temp. under stirring and stirred at about 60°C for 20 min; isolated by semi-preparative thin-layer chromy. (silica gel 60, petroleum ether/ethyl acetate 10:1); | A 40% B n/a |

-
-
7616-94-6
perchloryl fluoride

-
-
19282-10-1
Na(1+)*CBr(NO2)2(1-)=NaCBr(NO2)2

-
-
22632-20-8
fluoro bromo dinitromethane

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 36% |
| In N,N-dimethyl-formamide | 36% |
| In acetone | 36% |
-
-
7616-94-6
perchloryl fluoride

-
-
22632-20-8
fluoro bromo dinitromethane

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 36% |
| In N,N-dimethyl-formamide | 36% |
| In acetone | 36% |
-
-
7616-94-6
perchloryl fluoride

-
-
24280-12-4
bromo-dinitro-methane; potassium salt

-
-
22632-20-8
fluoro bromo dinitromethane

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 36% |
| In N,N-dimethyl-formamide | 36% |
| In acetone | 36% |
-
-
7616-94-6
perchloryl fluoride

-
-
19282-07-6
Na(1+)*CCl(NO2)2(1-)=NaCCl(NO2)2

-
-
19845-51-3
fluorochlorodinitromethane

| Conditions | Yield |
|---|---|
| In further solvent(s) in CH3O(CH2)2OCH3; | 33% |
| In N,N-dimethyl-formamide | 33% |
| In acetone | 33% |
Perchloryl Fluoride Chemical Properties
Chemistry informtion about Perchloryl Fluoride (CAS NO.7616-94-6) is:
IUPAC Name: Perchloryl Fluoride
Synonyms: Perchloryl Fluoride ; Chlorine Oxyfluoride ; Chlorine Trioxide Fluoride ; Chloryl Fluoroxide ; Fluorochlorine(Vii) Trioxide ; Hyperchloric Acid Fluoride
MF: ClFO3
MW: 70.4508032
EINECS: 231-526-0
Density 1.4 g/cm3
Melting point: −147.8 °C
Boiling point: −46.7 °C
Following is the molecular structure of Perchloryl Fluoride (CAS NO.7616-94-6) is:
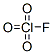
Perchloryl Fluoride Uses
Perchloryl Fluoride (CAS NO.7616-94-6) is used in organic chemistry as a mild fluorinating agent. It was the first industrially-relevant electrophilic fluorinating agent, used since the 1960s for producing fluorinated steroids.Perchloryl fluoride was investigated as a high performance liquid rocket fuel oxidizer. In comparison with chlorine pentafluoride and bromine pentafluoride, it has significantly lower specific impulse, but does not tend to corrode tanks. It does not require cryogenic storage.It can also be used in flame photometry as an excitation source.
Perchloryl Fluoride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LCLo | inhalation | 451ppm/4H (451ppm) | behavioral: convulsions or effect on seizure threshold lungs, thorax, or respiration: cyanosis gastrointestinal: nausea or vomiting | U.S. Army Chemical Warfare Laboratories. Vol. CWL, |
| guinea pig | LC50 | inhalation | 220mg/m3 (220mg/m3) | lungs, thorax, or respiration: "fibrosis, focal (pneumoconiosis)" blood: changes in erythrocyte (rbc) count blood: methemoglobinemia-carboxyhemoglobin | "Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 199, 1999. |
| mouse | LC50 | inhalation | 630ppm/4H (630ppm) | Pennsalt Chemicals Corp., Technical Div., New Products. | |
| rat | LC50 | inhalation | 385ppm/4H (385ppm) | Pennsalt Chemicals Corp., Technical Div., New Products. |
Perchloryl Fluoride Consensus Reports
Reported in EPA TSCA Inventory.
Perchloryl Fluoride Safety Profile
A poison gas which forms methemoglobin in the body and destroys red cells causing anemia, anorexia, and cyanosis. Recovery is said to be rapid, leaving no permanent physiological damage. Can be absorbed through the skin. Its odor can be detected as low as 10 ppm although this cannot be relied upon as an indication of toxic concentration in air. While non-flammable, it supports combustion. It is a powerful oxidizer. Moderately explosive. Potentially explosive reactions with combustible gases or vapors. benzene + aluminum trichloride, benzocyclobutene + butyllithium + potassium tert-butoxide, calcium acetylide, potassium cyanide, potassium thiocyanate, sodium iodide, charcoal, ethyl-4-fluorobenzoylacetate, hydrocarbons, hydrogen sulfide, nitrogen oxide, sulfur dichloride, vinylidene chloride, 3α-hydroxy-5β-androstane-11,17-dione-17-hydrazone, lithiated compounds, 2-lithio(dimethylamino-methyl)ferroxene, methyl-2-bromo-5,5-ethylene dioxy(2,2,1)bicycloheptane-7-carboxylate, aliphatic heterocyclic amines, sodium methoxide + methanol, vinylidene chloride. Reacts to form explosive products with nitrogenous bases (e.g., isopropylamine, isobutylamine, aniline, phenyl hydrazine, 1,2-diphenyl hydrazine), sawdust, lampblack. Violent reaction with finely divided organic materials. A fluorinating agent in chemical synthesis, and an oxidant in rocket fuel. When heated to decomposition it emits toxic fumes of F− and Cl−. See also FLUORINE and PERCHLORATES. Perchloryl fluoride is toxic, with a TLV of 3 ppm. It is a strong lung- and eye-irritant capable of producing burns on exposed skin. Its IDLH level is 385 ppm. Symptoms of exposure include dizziness, headaches, syncope, and cyanosis. Exposure to toxic levels causes severe respiratory tract inflammation and pulmonary edema.
RIDADR: 3157
HazardClass: 2.2
Perchloryl Fluoride Standards and Recommendations
OSHA PEL: TWA 3 ppm; STEL 6 ppm
ACGIH TLV: TWA 3 ppm; STEL 6 ppm
DOT Classification: 2.3; Label: Poison Gas, Oxidizer
Perchloryl Fluoride Analytical Methods
Perchloryl fluoride is produced primarily by the fluorination of perchlorates. Antimony pentafluoride is a commonly-used fluorinating agent:
ClO−4 + 3 HF + 2 SbF5 → FClO3 + H3O+ + 2 SbF−6
FClO3 reacts with alcohols to produce alkyl perchlorates, which are extremely shock-sensitive explosives.Using Friedel-Crafts catalysts, it can be used for introducing the –ClO3 group into aromatic rings via electrophilic aromatic substitution.
Perchloryl Fluoride Specification
Perchloryl Fluoride (CAS NO.7616-94-6) is a colorless, non corrosive gas with a characteristic sweet odor. Contact with the material may cause irritation to skin, eyes, and mucous membranes. Perchloryl fluoride is very toxic by inhalation and skin absorption. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket.It can slightly soluble in water. Perchloryl fluoride is a propellant and a powerful oxidant. It ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. It is toxic and may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution. It does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Related Products
- Perchloryl Fluoride
- Perchloryl Nitrite
- PERCHLORYL PERCHLORATE
- 7617-74-5
- 7617-93-8
- 76179-40-3
- 76180-96-6
- 76185-12-1
- 76185-65-4
- 76186-04-4
- 76188-55-1
- 76189-55-4
- 76189-56-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View