Synthetic route

| Conditions | Yield |
|---|---|
| In gas Irradiation (UV/VIS); photolysis by UV light at room temp.; | A 98.8% B n/a |
| In neat (no solvent, gas phase) Irradiation (UV/VIS); photolysis by UV light at room temp.; | A 98.8% B n/a |
| In gas Irradiation (UV/VIS); photolysis at 1-10 Torr; nearly complete decompn. within some h; | |
| In neat (no solvent, gas phase) Irradiation (UV/VIS); photolysis at 1-10 Torr; nearly complete decompn. within some h; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In neat (no solvent) Electrolysis; melt of KH2F3; passing through column with granuled NaF; | 95% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) passing over 1.0 g/min water-steam in 120 ml air at 1300°C for 30 minutes;; | 92.1% |
| In neat (no solvent) passing over 1.0 g/min water-steam in 120 ml air at 1300°C for 30 minutes;; | 92.1% |
| With silica gel In neat (no solvent) passing 1.0 g/min water-steam in 120 ml air over a mixture of 2.38 g CaF2 and 2.62 g SiO2 at 1300°C for 30 minutes;; | 90.5% |

| Conditions | Yield |
|---|---|
| In solid 1 equiv of K2NiF6 and ca. 3 equiv of BiF5 were premixed at ambient temp., F2 evolution started when the mixt. heated to about 60-70°C, peak pressure 990 Torr; | 75% |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| With silica gel In neat (no solvent) passing dry air over a mixture of 2.38 g CaF2 and 2.62 g SiO2 at 1300°C for 30 minutes;; | 52.9% |
| With SiO2 In neat (no solvent) passing dry air over a mixture of 2.38 g CaF2 and 2.62 g SiO2 at 1300°C for 30 minutes;; | 52.9% |
| With silica gel In neat (no solvent) passing dry air over a mixture of 2.38 g CaF2 and 2.62 g SiO2 at 1200°C for 30 minutes;; | 12.2% |

| Conditions | Yield |
|---|---|
| In solid 1 equiv of Cs2CuF6 and ca. 2 equiv of BiF5 were premixed at ambient temp., F2 evolution started when the mixt. heated to about 60-70°C, peak pressure 836 Torr; | 46% |
-
-
7787-62-4
bismuth pentafluoride

-
-
17218-47-2
K2NiF6

-
-
7783-63-3
titanium(IV) fluoride

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In solid equimolar amts. of reactants were premixed at ambient temp., F2 evolution started when the mixt. heated to about 60-70°C, peak pressure 820 Torr; | 45% |


| Conditions | Yield |
|---|---|
| In solid 1 equiv of Cs2MnF6 and 3 equiv of BiF5 were premixed at ambient temp., F2 evolution started when the mixt. heated to about 60-70°C, peak pressure 929 Torr; | 41% |

| Conditions | Yield |
|---|---|
| In solid equimolar amts. of reactants were premixed at ambient temp., F2 evolution started when the mixt. heated to about 60-70°C, peak pressure 810 Torr; | 14% |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; | |
| In melt Electrolysis; |

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of KF*1.6HF with 0.1-2,5% LiF at 100-110°C; current density: 0.097A/cm*cm; C-anode with 35-47% Cu, steel cathode;; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; KF*3HF (m.p.: 0-150°C); electrode: Fe, Ni, Cu or alloys of these metals; copper diaphragma;; | |
| In melt Electrolysis; electrolysis of KF*3HF; open or closed system; crucible of Cu; various cathode and anode; with or without diaphragma; various temp.;; | |
| In melt Electrolysis; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; closed crucible (cathode) and diaphragma of copper;; | |
| In melt Electrolysis; closed crucible (cathode) and diaphragma of copper;; | |
| In melt Electrolysis; electrolysis of KF*1.8HF in a closed crucible (cathode) of Cu at 160-250°C, 20 A and 8.5-9 V ; diaphragma of copper; graphite anode; current efficiency: 92-94%;; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of KF*2HF in a crucible (cathode) of monel metal at 73-75°C and 12 A; diaphragma of copper; Ni anode; current efficiency: 72%; sealed with Portland cement;; | |
| In melt Electrolysis; electrolysis of KF*2HF in a crucible (cathode) of monel metal at 73-75°C and 12 A; diaphragma of copper; Ni anode; current efficiency: 72%; sealed with Portland cement;; |

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of KF*2HF with 1% LiF and 2% NaF at 100°C, 300 A and 11 V; coal anode, steel cathode;; |

-
-
1279123-63-5
potassium hydrogenfluoride

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrochem. Process; laboratory fluorine cell (40 -42 % HF, 100 A, 80 - 100°C); passing through column with granulated NaF; | |
| In melt Electrolysis; graphite anode; description and drawing of apparatus given;; | |
| In melt Electrolysis; graphite anode; description and drawing of apparatus given;; |

| Conditions | Yield |
|---|---|
| In melt Electrolysis; KHF2 presaturated with HF (40 - 42 wt. %); adsorption on column packed with NaF pellets; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of KF*(2.9-6.7)HF at 15-50°C; various material for vessel, cathode and anode;; | |
| In melt Electrolysis; | |
| In melt Electrolysis; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; at 80-110°C;; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; |

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of a mixt. of KF*HF (65%) and NaF*HF (35%); vessel and diaphragma of Mg or Monel metal;; | |
| In melt Electrolysis; electrolysis of a mixt. of KF*HF (65%) and NaF*HF (35%); vessel and diaphragma of Mg or Monel metal;; | |
| In melt Electrolysis; electrolysis of a mixt. of KF*HF (65%) and NaF*HF (35%); vessel and diaphragma of Mg or Monel metal;; |

| Conditions | Yield |
|---|---|
| In water Electrolysis; reaction by use of aq. HF solns., mechanism discussed;; | |
| In water Electrolysis; reaction by use of aq. HF solns., mechanism discussed;; |


-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt byproducts: H2; Electrolysis; anode: 40% anthracite and 60% coke pressed at 1250-1300°C and 300-400 at; 70-85°C; 2000 A; pilot plant;; | |
| In melt Electrolysis; | |
| In melt Electrolysis; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of RbF; hollow C-anode (permeable to gas); current density: 0.0775 A/cm*cm; Cu-cathode;; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| In melt Electrolysis; electrolysis of KF; hollow C-anode (permeable to gas); current density: 0.0775 A/cm*cm; Cu-cathode;; |

| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrolysis; electrolysis of non-aq. HF and KF at -30 to -40°C;; | |
| In hydrogen fluoride HF (liquid); Electrolysis; electrolysis of non-aq. HF and KF at -30 to -40°C;; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| With oxygen In neat (no solvent) byproducts: Na4P2O7; heating of a mixt. of NaF and Na2P2O6 at 650-750°C in air;; | |
| With Na2P2O6; O2 In neat (no solvent) byproducts: Na4P2O7; heating of a mixt. of NaF and Na2P2O6 at 650-750°C in air;; |

| Conditions | Yield |
|---|---|
| With oxygen |


| Conditions | Yield |
|---|---|
| In hydrogen fluoride byproducts: H2; HF (liquid); Electrolysis; photoelectrolysis of anhydrous HF/NaF at a TiO(2-x)F(x) or n-TiO(2-x) electrode under 365 nm illumination or in the dark;; F2 identified by volatility at -196 °C, IR inactivity, MS and chemical behaviour;; |

| Conditions | Yield |
|---|---|
| With oxygen |


| Conditions | Yield |
|---|---|
| In neat (no solvent) formation at fluorination of SnO2 with F2 at 500-550°C;; | 100% |
| In neat (no solvent) formation at fluorination of SnO2 with F2 at 400°C;; | 80% |
| In neat (no solvent) reaction with fluorine at 500-550°C under formation of SnF4;; | |
| In neat (no solvent) reaction with fluorine at 500-550°C under formation of SnF4;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; all manipulations in metal vac. line; mixt. of calcd. amts. of metal powders reacted with 10% excess of F2 gas at 393 K for 3 h in steel autoclave, cooled to room temp.; excess F2 removed in dynamic vac., crystalline product scraped from upper surfaces of autoclave; elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; all manipulations in metal vac. line; mixt. of calcd. amts. of metal powders reacted with 10% excess of F2 gas at 393 K for 3 h in steel autoclave, cooled to room temp.; excess F2 removed in dynamic vac., crystalline product scraped from upper surfaces of autoclave; elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; all manipulations in metal vac. line; mixt. of calcd. amts. of metal powders reacted with 10% excess of F2 gas at 393 K for 3 h in steel autoclave, cooled to room temp.; excess F2 removed in dynamic vac., crystalline product scraped from upper surfaces of autoclave; elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) anhydrous CrF3 and F2 shaked for 29 h at 270-303 ° C; remaining F2 removed at -196 ° C; | 100% |
| addn. of F2 to CrF3 at -196°C, then 260°C for 65 h; | 92.5% |
| In neat (no solvent) High Pressure; CrF3 was loaded into a Monel autoclave in a dry-box; after evacuation F2 was admitted (30 atm); heating at 350°C for 2 h;; cooling to room temp.; autoclave was evacuated to 1E-1 torr for severalhours and subsequently opened in a dry-box; elem. anal.;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; all manipulations in metal vac. line; metal powder reacted with 10% excess of F2 gas at 393 K for 3 h in steel autoclave, cooled to room temp.; excess F2 removed in dynamic vac., crystalline product scraped from upper surfaces of autoclave; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| at 450℃; for 144h; Inert atmosphere; Milling; | 100% |


| Conditions | Yield |
|---|---|
| In hydrogen fluoride HF (liquid); dry Ar-atmosphere; metal reactor lined with fluorinated plastic, prepassivated with F2; 2 atm F2,(pptn. of AgF2 in one part), mechanical transfer of excess BiF5 onto AgF2, stirring (15 h); evapn., repeated treatment with BF3 (dissoln. of AgF2) and decantation,drying (dynamic vac.); | 99.7% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Xe and F2 condensed into nickel can (-196°C), warmed (room temp., pressure 34 atm.), preheated electric furnace (400°C) placed around the nickel can (7 h, pressure 78 atm.), quenched to room temp. in water, cooled (-78°C); excess Xe condensed into a storage cylinder (-196°C), evapn. (through a cold trap, -78°C); | 99.3% |
| In gas react. temp. of 400°C, Xe:F2 about 2.0, Monel vessel with volume between 95 and 1200 ml; cooling down with H2O;; | >95 |
| Electric Arc; react. of F2 with an excess of Xe in an electrical discharge; sublimation in vac., discarding the leading fraction; |

| Conditions | Yield |
|---|---|
| In gas byproducts: ClO2, ClF3; Irradiation (UV/VIS); pulsed irradiation of gaseous stoichiometric mixt. of F2 and Cl2 in a closed space at room temp. (light pulses lasting 5E-6 to E-3 s with λ=200-450 nm); impurities detected by spectroscopic methods; presence of ; | 99% |
| In neat (no solvent) equilibrium react. at 250°C;; | |
| In neat (no solvent) Cl2 and an excess of F2;; |

| Conditions | Yield |
|---|---|
| In hydrogen fluoride byproducts: O2, N2; HF (liquid); High Pressure; at 600°C for 1 h in an autoclave; evapd. at -196°C, condensed, sealed, recrystd. by cooling from 0 to -78°C; | 99% |

| Conditions | Yield |
|---|---|
| In trichlorofluoromethane Ar/F2 mixture bubbled into I2 in CFCl3 (at -45°C) until the I2 disappeared completely; solv. evapd., warmed (to -30°C), cooled (to -78°C); | 99% |

| Conditions | Yield |
|---|---|
| In hydrogen fluoride HF (liquid); HF added to Rh complex under vac., kept at -196°C, F2 added, stirred for ca. 2 h, further addn. of F2, stirred at room temp. for 1 h, excess F2 removed at -196°C, HF and SbF5 added, warmed to room temp., stirred at 70°C, CO added,; stirred at -20°C for 20 h; removal of all volatiles under vac.; | 99% |


| Conditions | Yield |
|---|---|
| rubidium fluoride equimolar amt. of educts, steel vessel, -78°C, yield: 97 mol-%; | 97% |
| potassium fluoride equimolar amt. of educts, steel vessel, -78°C, yield: 97 mol-%; | 97% |
| cesium fluoride equimolar amt. of educts, steel vessel, -78°C, yield: 97 mol-%; | 97% |

| Conditions | Yield |
|---|---|
| In gas Holloway's apparatus; continous working possible;; yields of 11gXeF4/h of very pure XeF4 are obtained;; | 97% |
| on heating of a Xe-F2 mixture (1:5) in a closed, 1600ml Ni vessel at 400°C with following chilling of react. vessel in water;; on pumping off of excess fluorine at -78°C and removing of resting Xe with U tube (with temp. of -195°C); in 1 hour 10g XeF4 with a yield >99% are obtained;; | >99 |
| Electrochem. Process; on electrical discharge at -78°C; detailed information about react. conditions and apparatus given;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: Cl2, O2; ClF added to CrO3, mixt. heated at 110°C for 4 h with periodic shaking, vessel evacuated at -196, -78°C, at room temp., vessel charged with F2, heated to 120°C for 12 h, removed F2 and ClO2F, repeated react. with F2 for 2 and 3 h; at -196°C removed excess F2, at room temp. removed ClO2F, elem. anal.; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: Cl2, O2; ClF added to CrO2F2, mixt. heated to 100°C for 12 h, vessel evacuated at -196, -78°C, at room temp., vessel charged with F2, heated to 120°C for 4 h; at -196°C removed excess F2, at room temp. removed ClO2F, elem. anal.; | A n/a B 97% |

-
-
117678-68-9
dodecacarbonyltetrairidium

-
-
7782-41-4
fluorine

-
-
146168-99-2, 146237-54-9
fac-iridiumtricarbonyltrifluoride

| Conditions | Yield |
|---|---|
| In hydrogen fluoride HF (liquid); (Schlenk, N2) to a soln. of Ir-complex in HF 2 atm of F2 at -78°Cwas added, the soln. was allowed to warm to room temp., stirred for 16 h; the soln. was removed in vac.; | 96% |
-
-
353-50-4
Carbonyl fluoride

-
-
7782-41-4
fluorine

-
A
-
373-91-1
hypofluorous acid trifluoromethyl ester

-
B
-
927-84-4
bis(trifluoromethyl)peroxide

| Conditions | Yield |
|---|---|
| yttrium(III) fluoride Kinetics; 150°C, 10 h; | A n/a B 95% |
| bismuth(III) fluoride Kinetics; 150°C, 12 h; | A n/a B 95% |
| terbium(III) fluoride Kinetics; 100°C, 30 h; | A n/a B 95% |


| Conditions | Yield |
|---|---|
| In gas at 119 - 232°C in Al-tubes about 30 - 45 min;; | A 95% B n/a |
| In gas at 170°C in Ni-tubes about 40 min;; | A 12% B n/a |
| nickel(II) fluoride In gas Kinetics; at 467, 497 and 530°K in Ni-tubes;; |

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| With polyacrylonitrile volume ratio of polyacrylnitrile:F2 = 1:60, at 20°C for 6 h then with F2 for 30 h; F2 dild. with He; | 95% |
| With polyacrylonitrile volume ratio of polyacrylnitrile:F2 = 1:60, at 20°C for 6 h then with F2 for 30 h; F2 dild. with He; | 95% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: BF3, TeF5OH, TeF6; under dry N2, B(OTeF5)3 in a Monel vessel evacd., cooled to -196°C, addn. of F2, closed vessel warmed to ambient temp., placed in an oven at 100°C, 67 h; cooled to -196°C, excess F2 pumped off, volatiles sepd. by fractional condensation during warming to room temp., TeF5OF retained in a trap cooled at -126°C; | 95% |
| In neat (no solvent) byproducts: BF3, TeF5OH, TeF6; under dry N2; B(OTeF5)3 in a Monel vessel evacd., cooled to -196°C, addn. of F2, closed vessel warmed to ambient temp., placed in an oven at 115°C, 24 h;; cooled to -196°C, excess F2 pumped off, volatiles sepd. by fractional condensation during warming to room temp., TeF5OF retained in a trap cooled at -126°C;; | 94% |
| In neat (no solvent) byproducts: BF3, TeF5OH, TeF6; under dry N2, B(OTeF5)3 in a stainless steel vessel evacd., cooled to -196°C, addn. of F2, closed vessel warmed to ambient temp., placed in an oven at 115°C, 51 h; cooled to -196°C, excess F2 pumped off, volatiles sepd. by fractional condensation during warming to room temp., TeF5OF retained in a trap cooled at -126°C; | 81% |

| Conditions | Yield |
|---|---|
| In hydrogen fluoride HF (liquid); Argon atmosphere, fluorocarbon polymer tube, addn. of fluorine (5 h, 800Torr), vigorous agitation (20°C, 21 h); decantation, washing (HF); | 95% |

-
-
168098-42-8
deca-B-methyl-1,12-dicarba-closo-dodecaborane(12)

-
-
7782-41-4
fluorine

-
-
356060-61-2
perfluro-deca-B-methyl-para-carborane

| Conditions | Yield |
|---|---|
| In further solvent(s) a soln. (perfluorononane) of B compd. pumped into reactor with N2/F2 bubbling, gradually heated to 110°C (24 h), held for 4 d; F2 turned off, allowed to purge for 4 h, filtered, solvent removed (vac.), sublimated (65°C/0.01 mm); | 94% |


| Conditions | Yield |
|---|---|
| In water byproducts: HF; soln. NaSO3NH2 (H2O) placed into teflon-FEP ampule; cooling to 0°C, F2 (10% v/v in N2) introduced; 45 min of fluorination; mixt. pumped to dryness at 0°C; | 94% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0-5°C 1.5 h; | 92.3% |
| With NaOH In water at 0-5°C 1.5 h; | 92.3% |
| In water 40% F2 in N2; velocity of flow 9l/h; at 0-5°C; | 90% |
-
-
1279123-63-5
potassium hydrogenfluoride

-
-
60-29-7
diethyl ether

-
-
7664-39-3
hydrogen fluoride

-
-
7782-41-4
fluorine

| Conditions | Yield |
|---|---|
| Stage #1: potassium hydrogenfluoride; 12-ammoniocarba-closo-dodecaborane; hydrogen fluoride; fluorine at -78 - 20℃; Stage #2: diethyl ether In hexane | 92% |

Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View




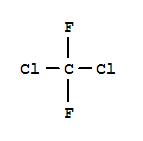


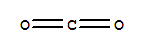






 C,
C, T+
T+