Chemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:7782-44-7
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: Oxygen Synonyms: Dioxygen;hyperoxia;liquidoxygen;Liquid-oxygen-;lox;Molecular oxygen;molecularoxygen;molekularerSauerstoff CAS: 7782-44-7 MF: O2 MW: 32 EINECS: 231-956-9 Product Categories: refrigerants;Inorganics;Analytical Rea
Cas:7782-44-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryKono Chem Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air Port:Beijing or Guangzhou
Zhuozhou Wenxi import and Export Co., Ltd
Product Description Description & Specification Category Pharmaceutical Raw Materials, Fine Chemicals, Bulk drug Standard Medical standard
Cas:7782-44-7
Min.Order:1 Kilogram
FOB Price: $112.0
Type:Trading Company
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Wuhan ZeShanCheng Biomedical Technology Co., Ltd.
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of highl
HENAN SUNLAKE ENTERPRISE CORPORATION
Our Services 1.Certificate Of Analysis (COA) 2.Material Safety Data Sheet (MSDS) 3.Route of synthesis (ROS) 4.Method of Aanlysis (MOA) 5.Nuclear Magnetic Resonance (NMR) 6.Packing pictures and loading video before loading 7.Free S
Anqiu Hengan Gas Manufacture Factory
-oxygen gas -99.5% purity -seamless steel oxygen gas cylinders package -filling pressure max up to 200bar -factory price Appearance:cylinder pack Package:cylinder pack Application:for industrial use Transportation:by sea Port:Qingdao Port
Hangzhou Sinopoly AS manufacturing Co.,Ltd
Technical Features Low energy consumption, less cost, strong adaptability, rapid nitrogen production and adjustable purity; Perfect technique process design and optimal using effect; Modular structure design and saving of floor space; Con
Chengdu Taiyu Industrial Gases Co., Ltd.
Oxygen O2 CAS No.: 7782-44-7 EINECS No.: 231-956-9 UN No.: UN1072 Purity: 99.995%-99.9997% Dot Class: 2.2 Appearance: Colorless Grade Standard: Industrial Grade, Medical Grade, Electronic Grade.
Shanghai united Scientific Co.,Ltd.
United Scientific Company Located in Shanghai of China , is a competitive player in the global specialty and fine chemical market. Fenghua has both the expertise and flexibility to produce a wide range of chemicals. Focusing on developing the innovat
Shaanxi Mingqi Chemical Co., Ltd
Known for its best quality and competitve price, this chemicals we offered is widely appreciated by our customers. Our advantages:1, High quality with competitive price:1) Standard:BP/USP/EP/Enterprise standard2) All Purity≥99%3) We are manufacturer
GZ HONESTCHEM CO., LTD
OxygenAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air
Nanjing Raymon Biotech Co., Ltd.
dioxygen Storage:Store in dry and cool condition Package:25kg or according to cutomer's demand Application:Chemical research/Pharmaceutical intermediates Transportation:By Sea,by Air,By courier like DHL or Fedx. Port:Shanghai/Shenzhen
Synova( Tianjin ) Chemical Technology Co,.Ltd
The company have effective management team, professional technical R & D personnel, the service spirit of customer oriented. We have long-term cooperation with famous domestic manufacturer, and excellent customer resources overseas. We are skilled in
Skyrun Industrial Co.,Ltd
Good Price, high main content Application:Medical intermediate; Used in organic synthesis
Hebei Ruishun Trade Co.,Ltd
Supply top quality products with a reasonable price Application:api
yuyongmei
YUYONGMEI was established in Aug,1999, located in the industrial park of Nanjing University of Technology, It is a private enterprises in Jiangsu Province with 1200 square meters’ R&D center. Our R&D center has a well-equipped synthetic laboratory a
NovaChemistry
high purity Application:Drug intermediates Materials intermediates and active molecules
Societa Italiana Acetilene & Derivati S.p.A.
Appearance:colourless gas
Synthesia, a.s.
more information,pls contact with us!
Synthetic route

-
B
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| With perchloric acid; oxygen In acetonitrile Kinetics; | A 100% B n/a |
-
-
75-15-0
carbon disulfide

-
-
7722-84-1
dihydrogen peroxide

-
-
80937-33-3
oxygen

-
A
-
7446-09-5
sulfur dioxide

-
B
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In gas Kinetics; byproducts: HS; other Radiation; H2O2 is photolysed at 266 nm (Nd:YAG laser) in a flow reactor, addn. of CS2 (NO) and O2 (N2); HO2 yield measurement by LMR (laser magnetic resonance), SO2 yield measurement by CIMS (chemical ionization mass spectometry); | A 90% B 95% |


| Conditions | Yield |
|---|---|
| With synthetic air; oxygen In gaseous matrix Kinetics; C2H4, CO and synthetic air mixture addn. into O3/O2 flow; | 66% |


| Conditions | Yield |
|---|---|
| With synthetic air; oxygen In gaseous matrix Kinetics; byproducts: hydroxyethylperoxy radical; C2H4 (1-3 ml/min) and synthetic air (3 ml/min) mixture addn. into O3/O2 flow; | A 20% B 39% |

-
-
3352-57-6
hydroxyl

-
-
14989-30-1
chlorine monoxide

-
A
-
7647-01-0
hydrogenchloride


-
C
-
80937-33-3
oxygen

-
D
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In gas Kinetics; between 218 and 298 K, OH source: F + H2O or H + NO2, ClO source: Cl + O3, reaction carried out in a discharge-flow system; | A 9% B n/a C n/a D n/a |
| In gaseous matrix Kinetics; carrier gas: He; hydroxyl radicals were prepared by the reaction between H and NO2; ClO radicals were prepared by the reaction between O3 an Cl; resonance-fluorescence measurements; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; byproducts: C2H4, C2H5O2; Irradiation (UV/VIS); reaction of O2 with C2H5 radicals formed by flash-light photolysis (22 °C, 5-100 Torr total pressure); 99.9% of C2H5 reacted with formation of C2H5O2; rate constant;; | 0.1% |
| In neat (no solvent) Kinetics; byproducts: C2H4; reaction of C2H5 radicals with O2 at T<320 °C; mechanism;; |

| Conditions | Yield |
|---|---|
| In gas Kinetics; CH4-O2 reaction at 1100 °C and total pressure of 10 Torr; HO2 decay (1. order);; mass spectroscopy;; | |
| In neat (no solvent) CH4-O2 flames (12 Torr);; mass spectroscopy; assignment to HO2 is doubtful;; | |
| In neat (no solvent) CH4-O2 flames (70 Torr);; mass spectroscopy;; | |
| In neat (no solvent) byproducts: CH3, H2O2; reaction of 5 Torr CH4 with 3 Torr O2 at 1090 °C (reaction period 10E-2 s); collision yield; following reaction;; mass spectroscopy;; | 0.05% |
| In neat (no solvent) examination of the CH4-O2 reaction;; |

| Conditions | Yield |
|---|---|
| Kinetics; byproducts: OH, H2O, O2; at 298-386 K and 15-150 Torr; | |
| In neat (no solvent) Kinetics; byproducts: OH, H2O, O2; gaseous H2O2 was mixed with O+O2; rate constant;; | |
| In neat (no solvent) |

| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; reaction of OH (produced on reaction of H + NO2 -> OH + NO) and O3 (He carrier gas, 1.5 - 4 Torr, 300 K - 423 K); |


| Conditions | Yield |
|---|---|
| With water; acetic acid In water Kinetics; byproducts: O2; Irradiation (UV/VIS); | |
| In neat (no solvent) Kinetics; byproducts: O2; OH radicals formed from H+NO2 reacted with O3; rate constant;; UV absorption measurements;; |

| Conditions | Yield |
|---|---|
| With nitrogen In gas Kinetics; kinetic of react. of OH radical with H2O2 studied over temp. range 96-296 K; H2O2/N2 gas expanded through Laval nozzle; laser-induced fluorescence of OH radical used as probe; | |
| In gas Kinetics; kinetics studied at 250-1250 K by means of UV spectroscopy; | |
| In gaseous matrix Kinetics; 245-423 K; total pressure=45-500 torr; in He stream, in contact with quarz, Pyrex or Teflon only; not sepd.; |


| Conditions | Yield |
|---|---|
| In gas fast-flow discharge system (according to: T. E. Kleindienst, B. J. Finlayson-Pitts, J. Chem. Phys. Lett. 61 (1979) 300), halocarbon wax or boric acid coated flow tube, H or O3 excess; resonance fluorescence technique, titration of H; |


| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; other Radiation; laser photolysis (mixt. of O3 with He (1:1E+4), KrF excimer laser); detection by time-resolved atomic absorption spectroscopy; | |
| fast-flow discharge system (room temp., excess of O3, bychoise addn. ofCO or NO); |

-
-
7722-84-1
dihydrogen peroxide

-
-
10028-15-6
ozone

-
A
-
3352-57-6
hydroxyl

-
B
-
80937-33-3
oxygen

-
C
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In water Kinetics; |

-
-
7732-18-5
water

-
A
-
3352-57-6
hydroxyl

-
B
-
1333-74-0
hydrogen

-
C
-
7722-84-1
dihydrogen peroxide

-
D
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| Kinetics; other Radiation; effect of radiation type and scavanger concn. in water radiolysis; |

-
-
7783-06-4
hydrogen sulfide

-
-
7732-18-5
water

-
-
80937-33-3
oxygen

-
A
-
19936-25-5
sulfur dioxide hydrate

-
B
-
95690-30-5
SO3 * water

-
C
-
7664-93-9
sulfuric acid

-
D
-
7446-09-5
sulfur dioxide

-
E
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In solid matrix Irradiation (UV/VIS); further products; 270-420 nm photolysis in O2-matrix at 13 K (4-14 h); product ratio depending on matrix composition; not isolated, detected by IR; |

-
-
7732-18-5
water

-
-
80937-33-3
oxygen

-
A
-
155306-81-3
hydrogen peroxide water

-
B
-
7722-84-1
dihydrogen peroxide

-
C
-
10028-15-6
ozone

-
D
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In solid matrix Irradiation (UV/VIS); photolysis at 13 K for 1.8 h (λ > 220 nm); not isolated, detected by IR; |

-
-
74-90-8
hydrogen cyanide

-
A
-
75-13-8
isocyanic acid

-
C
-
2074-87-5
carbon nitride

-
D
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| Ar/HCN is codeposited with O atoms (produced by microwave discharge of Ar/O2)on cold CsI window; IR matrix isolation study; |

-
-
14701-21-4
silver (I) ion

-
-
14691-59-9
hydroperoxide anion

-
A
-
7440-22-4
silver

-
B
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In water mechanism of H2O2-decomposition discussed;; | |
| In water mechanism of H2O2-decomposition discussed;; |

-
-
7722-84-1
dihydrogen peroxide

-
-
16518-46-0
carbonate radical anion

-
A
-
71-52-3
hydrogen carbonate

-
B
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In water pH=6.3, 24°C; |


| Conditions | Yield |
|---|---|
| In gaseous matrix byproducts: HCl; He carrier gas; |

| Conditions | Yield |
|---|---|
| With oxygen In gas Kinetics; Flash photolysis-shock tube react.of O2/Ar-mixt. with H (produced by photodissociation of NH3 or H2O).; Rate consts. between 746 and 1705 K at various pressures given.; |


| Conditions | Yield |
|---|---|
| In gas Kinetics; under Ar, mixed, reacted at 293 K (P=1 kPa); ESR, gas chromy.; |


| Conditions | Yield |
|---|---|
| In gas Kinetics; rate coefficients measured via OH absorption behind reflected shock waves at 950-3100 K; H2/O2/Ar mixtures prepared manometrically and allowed to stand for at least 48 h; |


| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; H and O2 reacted in excess of Ar in fast flow-discharge tube at 2-10 Torr and at 231-512 K; detected by mass spectrometry and GC chromy; | |
| With He or N2 or H2O In gas Kinetics; other Radiation; reacting H atoms with O2 in the presence of He, N2 or H2O (microwave discharge, pressure 0.25 - 1 Torr); | |
| With nitrogen In gas Kinetics; |

| Conditions | Yield |
|---|---|
| Kinetics; in O2/H2 flame; fluorescence spectroscopy; |


| Conditions | Yield |
|---|---|
| With isopropyl alcohol; 12-tungstoferric acid In water Kinetics; Irradiation (UV/VIS); soln. contg. heteropolytungstate ion (2E-6 M) and i-PrOH (1.7 M) subjected to 10E-6 s photoflash in presence of O2 (2E-4 M) at pH 1.0 (0.05 M H2SO4); | |
| With isopropyl alcohol; sodium phosphotungstate In water Kinetics; Irradiation (UV/VIS); soln. contg. heteropolytungstate ion (2E-6 M) and i-PrOH (1.7 M) subjected to 10E-6 s photoflash in presence of O2 (2E-4 M) at pH 1.0 (0.05 M H2SO4); | |
| With isopropyl alcohol; sodium metatungstate In water Kinetics; Irradiation (UV/VIS); soln. contg. polytungstate ion (2E-6 M) and i-PrOH (1.7 M) subjected to10E-6 s photoflash in presence of O2 (2E-4 M) at pH 1.0 (0.05 M H2SO4); | |
| With isopropyl alcohol; 12-tungstosilicic acid, K-salt In water Kinetics; Irradiation (UV/VIS); soln. contg. heteropolytungstate ion (2E-6 M) and i-PrOH (1.7 M) subjected to 10E-6 s photoflash in presence of O2 (2E-4 M) at pH 1.0 (0.05 M H2SO4); |
-
-
2597-44-6
formyl radical

-
-
80937-33-3
oxygen

-
A
-
201230-82-2
carbon monoxide

-
B
-
7782-44-7
hydroperoxyl radical

-
-
7783-06-4
hydrogen sulfide

-
-
80937-33-3
oxygen

-
A
-
7664-93-9
sulfuric acid

-
B
-
7446-09-5
sulfur dioxide

-
C
-
7446-11-9
sulfur trioxide

-
D
-
10028-15-6
ozone

-
E
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| byproducts: H2O*SO2, H2O*SO3; Irradiation (UV/VIS); in solid O2, 15K; H2O*SO2 and H2O*SO3 present in significant amounts; detected by IR; |

-
-
7783-06-4
hydrogen sulfide

-
-
80937-33-3
oxygen

-
A
-
19936-25-5
sulfur dioxide hydrate

-
B
-
95690-30-5
SO3 * water

-
C
-
7664-93-9
sulfuric acid

-
D
-
7446-09-5
sulfur dioxide

-
E
-
7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In solid matrix Irradiation (UV/VIS); further products; 270-420 nm photolysis in O2-matrix at 13 K (4-14 h); product ratio depending on matrix:solute ratio (200-8000); not isolated, detected by IR; |


| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; Cl concn. 3E11 - 3E12 molecule/cm3, HO2 concn. 1E10 - 5E11 molecule/cm3,236 K, 1 Torr of He; | A 94.5% B n/a |
| In gaseous matrix Kinetics; Cl concn. 3E11 - 3E12 molecule/cm3, HO2 concn. 1E10 - 5E11 molecule/cm3,256 K, 1 Torr of He; | A 79.4% B n/a |
| In gaseous matrix Kinetics; Cl concn. 3E11 - 3E12 molecule/cm3, HO2 concn. 1E10 - 5E11 molecule/cm3,276 K, 1 Torr of He; | A 74.8% B n/a |
| In gaseous matrix Kinetics; Cl concn. 3E11 - 3E12 molecule/cm3, HO2 concn. 1E10 - 5E11 molecule/cm3,296 K, 1 Torr of He; | A 70.4% B n/a |


| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; Cl concn. 2E10 - 5E10 molecule/cm3, HO2 concn. 4E11 - 4E12 molecule/cm3,336 K, 1 Torr of He; | A 26% B n/a |
| In gaseous matrix Kinetics; Cl concn. 2E10 - 5E10 molecule/cm3, HO2 concn. 4E11 - 4E12 molecule/cm3,316 K, 1 Torr of He; | A 23.6% B n/a |
| In gaseous matrix Kinetics; Cl concn. 2E10 - 5E10 molecule/cm3, HO2 concn. 4E11 - 4E12 molecule/cm3,296 K, 1 Torr of He; | A 18.6% B n/a |


| Conditions | Yield |
|---|---|
| Kinetics; byproducts: O2; rate constant;; | |
| In sulfuric acid Kinetics; byproducts: O2; other Radiation; decrease of HO2 which was formed by pulsed or continuous electron radiation of H2O2-O2-H2SO4 soln.; rate constant;; | |
| In gaseous matrix Kinetics; byproducts: O2; 298 K, He or Ar present at total pressures 75 - 730 torr; |

| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; reaction of O2H (produced on reaction of H + O2 + M -> HO2 + M) and O3 (He carrier gas, 1.5 - 4 Torr, 245 K - 365 K); | |
| In gas Kinetics; discharge-flow system at room temp.;; | |
| In gas Kinetics; rate constant determined in the temp. range 233-400 K; |


| Conditions | Yield |
|---|---|
| byproducts: OH; |

| Conditions | Yield |
|---|---|
| In water |

| Conditions | Yield |
|---|---|
| Kinetics; in O2/H2 flame; fluorescence spectroscopy; |


| Conditions | Yield |
|---|---|
| Kinetics; in O2/H2 flame; fluorescence spectroscopy; |


| Conditions | Yield |
|---|---|
| Kinetics; in O2/H2 flame; fluorescence spectroscopy; |


| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; byproducts: O2; react. of atomic oxygen and HO2 radicals at 2 Torr, 299 K in a He flow; not isolated, detected by resonance fluorescence technique; | |
| In gaseous matrix Kinetics; byproducts: O2; educts were produced by laser pulsed photolysis of O3 and H2O2 in N2 or Ar at 10-500 Torr; detection by time-resolved fluorescence; | |
| In neat (no solvent) Kinetics; byproducts: O2; rate constant;; |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
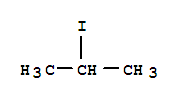
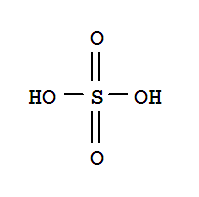













 O,
O, C
C