-
Name
peroxomonosulphuric acid
- EINECS
- CAS No. 7722-86-3
- Article Data82
- CAS DataBase
- Density 2.239g/cm3
- Solubility
- Melting Point 45, decomposing [HAW93]
- Formula H2O5S
- Boiling Point
- Molecular Weight 114.079
- Flash Point
- Transport Information
- Appearance
- Safety Strong irritant. Powerful oxidizer. An explosive. Explosive reaction acetone; alcohols; aromatics (e.g., aniline; benzene; phenol); platinum; manganese dioxide; silver. Incompatible with acetone; catalysts; fibers. When heated to decomposition it emits toxic fumes of SOx. See also PEROXIDES, INORGANIC; PEROXIDES, ORGANIC.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Peroxomonosulphuric acid;
- PSA 79.44000
- LogP 0.13700
Synthetic route

| Conditions | Yield |
|---|---|
| in traces H2O2, Cl2, H2S2O8; | 96.6% |
| in traces H2O2, Cl2, H2S2O8; | 96.6% |
-
-
50981-12-9, 10193-30-3
sulfuric acid monohydrate

-
A
-
7722-86-3
caro's acid

-
B
-
7722-84-1
dihydrogen peroxide

-
C
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| without water, -10°C; | A 93.5% B 1.4% C 5.1% |
| without water, -10°C; | A 93.5% B 1.4% C 5.1% |


| Conditions | Yield |
|---|---|
| With H2SO4 In neat (no solvent) 87.3% H2O2 added to 65% oleum at 0-10°C within 1 h; crystals isolated by slow cooling to -20°C for 24 h; | 62.9% |
| In neat (no solvent) reaction with anhydrous H2O2, cooling;; | |
| under heat evolution, in water proof condition forming very little product; | |
| In water -5°C; |

| Conditions | Yield |
|---|---|
| With ozone In water | A n/a B 1% |
| With ozone In water | A n/a B 1% |
| With ozone ozonisated H2SO3 soln.; | A n/a B <1 |
| With ozone ozonisated H2SO3 soln.; | A n/a B <1 |
-
-
7446-11-9
sulfur trioxide

-
-
7732-18-5
water

-
-
7722-84-1
dihydrogen peroxide

-
A
-
7722-86-3
caro's acid

-
B
-
7664-93-9
sulfuric acid

-
C
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| at 13 - 15℃; for 5.5 - 7h; |


| Conditions | Yield |
|---|---|
| In water Kinetics; byproducts: H2O; slow addn. of H2O2 to H2SO4 soln.; react. carried out in temp. range 283-323 K, thermostated; titrimetry; | |
| In not given concd. H2SO4; | |
| In water at 2 - 8℃; for 3.5h; Product distribution / selectivity; | |
| at 92℃; Product distribution / selectivity; Cooling; | |
| In not given concd. H2SO4; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) cooling;; | |
| >98.5 | |
| >98.5 |
-
-
7446-11-9
sulfur trioxide

-
-
7722-84-1
dihydrogen peroxide

-
A
-
7722-86-3
caro's acid

-
B
-
7664-93-9
sulfuric acid

| Conditions | Yield |
|---|---|
| In sulfuric acid addition of H2O2 (100%) to oleum;; |


| Conditions | Yield |
|---|---|
| With ozone In water | <1 |
| In water Electrolysis; anodic process; | |
| In not given | |
| With O3 In water | <1 |
-
-
7664-93-9
sulfuric acid

-
A
-
7722-86-3
caro's acid

-
B
-
7722-84-1
dihydrogen peroxide

-
C
-
80937-33-3
oxygen

-
D
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| In water Electrolysis; glow discharge electrolysis by alternating current; mechanismus discussed; | |
| In water Electrolysis; glow discharge electrolysis by alternating current; 12°C; C(val) H2SO4 = 1-10; 50 Hz; current density 0.04 A/cm2; p = 11 torr; | |
| In water Electrolysis; glow discharge electrolysis by alternating current; 12°C; C(val) H2SO4 = 1-10; 50 Hz; current density 0.04 A/cm2; p = 11 torr; | |
| In water Electrolysis; glow discharge electrolysis by alternating current; mechanismus discussed; | |
| In water Electrolysis; glow discharge electrolysis by alternating current; mechanismus discussed; |

-
-
7664-93-9
sulfuric acid

-
A
-
7722-86-3
caro's acid

-
B
-
7722-84-1
dihydrogen peroxide

-
C
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| In water Electrolysis; glow discharge electrolysis; 10°C; C(val) H2SO4 = 0.5-4 as well as 36% H2SO4; | |
| In water Electrolysis; glow discharge electrolysis; ambient temp. and -50°C; 11-55 % H2SO4 ; 13 torr; | |
| Electrolysis; anod in gas-filled room, alternating or continuous current, glow discharge; |


| Conditions | Yield |
|---|---|
| With sulfuric acid In not given cooling;; | |
| With sulfuric acid In water byproducts: H2O; | |
| With sulfuric acid In not given byproducts: H2O; |
-
-
7790-94-5
chlorosulfonic acid

-
-
7722-84-1
dihydrogen peroxide

-
A
-
7722-86-3
caro's acid

-
B
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| In not given slow addition of H2O2 to HOSO2Cl, cooling; ratio of products depending on amount of used H2O2;; | |
| In not given | |
| In not given |

-
-
13445-49-3
Marshall's acid

-
-
7722-86-3
caro's acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In neat (no solvent) product crystallises; | |
| With HNO3 hydrolysis; | |
| With H2O2 In neat (no solvent) product crystallises; |

| Conditions | Yield |
|---|---|
| With water In not given byproducts: H2O2; | |
| In water during storage of H2SO4 solns. having been previously electrolysed; | |
| With H2O In not given byproducts: H2O2; |

| Conditions | Yield |
|---|---|
| hydrolysis; | |
| hydrolysis; |


| Conditions | Yield |
|---|---|
| hydrolysis, faster with concd. than with dild. H2SO4; | |
| 40% H2SO4; | >99 |

| Conditions | Yield |
|---|---|
| In not given | A 0% B 0% |


-
-
7722-86-3
caro's acid

| Conditions | Yield |
|---|---|
| With sulfuric acid concd. H2SO4; | |
| With H2SO4 concd. H2SO4; |

| Conditions | Yield |
|---|---|
| With water In sulfuric acid byproducts: H2O2; effect of H2SO4 concn. on the H2O2/H2SO5 ratio; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; water In water Kinetics; byproducts: O2; with over 15% H2SO4 ; at 98 °C; | |
| With H2SO4; H2O In water Kinetics; byproducts: O2; with over 15% H2SO4 ; at 98 °C; |

-
-
7722-86-3
caro's acid

| Conditions | Yield |
|---|---|
| With water In water no hydrolysis; evidence by a number of reacts., which do not take place;; | 0% |
-
-
7664-93-9
sulfuric acid

-
-
7446-11-9
sulfur trioxide

-
A
-
7722-86-3
caro's acid

-
B
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| In neat (no solvent) reaction by influence of a O3-O2 mixture on fuming H2SO4 (with a content of free SO3 between 38.21-76.59%);; | |
| In neat (no solvent) reaction by influence of a O3-O2 mixture on fuming H2SO4 (with a content of free SO3 between 38.21-76.59%);; |


| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| In not given -10°C; with the exclusion of water; | A n/a B >99 |
| In not given -10°C; with the exclusion of water; | A n/a B >99 |


| Conditions | Yield |
|---|---|
| Ba-peroxodisulfat /containing crystal water/ standing for a week; |
-
-
14996-02-2
hydrogensulfate

-
A
-
7722-86-3
caro's acid

-
B
-
7789-21-1
fluorosulphonic acid

-
C
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| With water; fluorine In water |


| Conditions | Yield |
|---|---|
| In water Kinetics; Electrolysis; effect of electrol. duration discussed; 14.1 n-H2SO4; 7°C; 0.75 A/cm2; Pt anod; | |
| With ozone In not given fuming H2SO4; | |
| In water Kinetics; Electrolysis; 15 n-H2SO4; 30 A/dm2; |

| Conditions | Yield |
|---|---|
| byproducts: ozone; under cooling, concd. H2SO4; | |
| byproducts: ozone; under cooling, concd. H2SO4; |
-
-
7722-86-3
caro's acid

-
-
3736-92-3
4-[2-(phenylthio)ethyl]-1,2-diphenyl-3,5-pyrazolidinedione

-
-
57-96-5
4-[2-(phenylsulfinyl)ethyl]-1,2-diphenyl-3,5-pyrazolidinedione

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium hydrogensulfite In water; acetic acid; ethyl acetate; toluene | 60% |
-
-
7722-86-3
caro's acid

-
-
13445-49-3
Marshall's acid

| Conditions | Yield |
|---|---|
| decompn. during storage (8 days); | 15.3% |
| With chlorosulfonic acid | |
| With HSO3Cl |

| Conditions | Yield |
|---|---|
| In water at 11 - 17℃; for 0.3h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| In water |
-
-
7722-86-3
caro's acid

-
-
120131-05-7
fluoromalonaldehyde bis(diethyl acetal)

-
-
685-88-1
fluoromalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With ammonium persulfate; sulfuric acid In ethanol; water |
-
-
7722-86-3
caro's acid

-
-
10028-15-6
ozone

| Conditions | Yield |
|---|---|
| In not given reaction by let standing of 92% concd. H2SO5 soln. shortly above 0 °C;; not isolated, detection by odor;; | |
| In sulfuric acid decompn.; | |
| In neat (no solvent) spontaneous decompn.;; |

| Conditions | Yield |
|---|---|
| With sulfur In sulfuric acid | |
| With sulfur dioxide; water In water |

| Conditions | Yield |
|---|---|
| With water In sulfuric acid hydrolysis faster in highe concn.; | |
| With H2O hydrolysis; |
-
-
7722-86-3
caro's acid

-
-
80937-33-3
oxygen

| Conditions | Yield |
|---|---|
| Irradiation (UV/VIS); blue light; 98.6°C, decomp. in 24 h; | |
| platinum in presence of H2O2 faster than alone or H2O2 without Pt; | |
| With potassium permanganate |
-
-
7722-86-3
caro's acid

-
-
10058-23-8
oxone

| Conditions | Yield |
|---|---|
| With KOH In ethanol |
-
-
7722-86-3
caro's acid

-
-
29683-94-1
hydroxyperoxide

| Conditions | Yield |
|---|---|
| In sulfuric acid aq. sulfuric acidic soln. of Caro's acid;; | 0% |
| In sulfuric acid aq. sulfuric acidic soln. of Caro's acid;; | |
| In sulfuric acid aq. H2SO4; aq. sulfuric acidic soln. of Caro's acid;; | 0% |
| In sulfuric acid aq. H2SO4; aq. sulfuric acidic soln. of Caro's acid;; |

| Conditions | Yield |
|---|---|
| With pyrographite In sulfuric acid pulverised charcoal, heating; | |
| With charcoal In sulfuric acid pulverised charcoal, heating; |

Peroxymonosulfuric Acid Chemical Properties
Chemistry informtion about Peroxymonosulfuric Acid (CAS NO.7722-86-3) is:
IUPAC Name: Tetraoxathiolane 5,5-Dioxide
Synonyms: Peroxomonosulphuric Acid ; Peroxysulfuric Acid ; Persulfuric Acid ; Sulfomonoperacid ; Peroxymonosulfuric Acid ; Caro Acid ; Peroxosulfuric Acid
MF: O6S
MW: 128.0614
EINECS: 231-766-6
Density: 1.7-1.8 g/cm3
Following is the molecular structure of Peroxymonosulfuric Acid (CAS NO.7722-86-3) is:
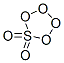
Peroxymonosulfuric Acid History
Peroxymonosulfuric Acid was first described by Heinrich Caro, after whom it is named.
Peroxymonosulfuric Acid Uses
Peroxymonosulfuric Acid (CAS NO.7722-86-3) has been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. Alkali metal salts of H2SO5 show promise for the delignification of wood. Ammonium, sodium, and potassium salts of Peroxymonosulfuric Acid are used in the plastics industry as polymerization initiators, etchants, desizing agents, soil conditioner, and for decolorizing and deodorizing oils. Potassium peroxymonosulfate, KHSO5, is the potassium acid salt of peroxymonosulfuric acid. It is widely used as an oxidizing agent.
Peroxymonosulfuric Acid Safety Profile
Strong irritant. Powerful oxidizer. An explosive. Explosive reaction acetone; alcohols; aromatics (e.g., aniline; benzene; phenol); platinum; manganese dioxide; silver. Incompatible with acetone; catalysts; fibers. When heated to decomposition it emits toxic fumes of SOx. See also PEROXIDES, INORGANIC; PEROXIDES, ORGANIC.
RIDADR: 1483
HazardClass: 5.1
PackingGroup: II
Peroxymonosulfuric Acid Analytical Methods
The laboratory scale preparation of Caro's acid involve the combination of chlorosulfuric acid and hydrogen peroxide.
H2O2 + ClSO2OH → H2SO5 + HCl
Large scale production of Caro's acid is usually done on site, due to its instability. According to the patent by Martin, Caro's acid is produced by reacting >85% sulfuric acid and >50% hydrogen peroxide ("Piranha solution").
H2O2 + H2SO4 → H2SO5 + H2O
Peroxymonosulfuric Acid Specification
Peroxymonosulfuric Acid (CAS NO.7722-86-3) is H2SO5, a liquid at room temperature. In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO-O-S(O)2-OH. It is sometimes confused with H2S2O8, known as peroxydisulfuric acid. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO-S(O)2-O-O-S(O)2-OH. As with all strong oxidizing agents, Peroxymonosulfuric Acid should be kept away from organic compounds such as ethers and ketones because of its ability to peroxidize the compound, creating a highly unstable molecule such as acetone peroxide.
Related Products
- Peroxymonosulfuric Acid
- 77228-67-2
- 7722-88-5
- 772311-98-5
- 7723-14-0
- 772-31-6
- 77232-38-3
- 772327-44-3
- 77-23-6
- 772-38-3
- 77238-31-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View