-
Name
Phenolphthalein
- EINECS 201-004-7
- CAS No. 77-09-8
- Article Data45
- CAS DataBase
- Density 1.385 g/cm3
- Solubility Water: <0.1 g/100 mL
- Melting Point 258-263 °C
- Formula C20H14O4
- Boiling Point 557.8 °C at 760 mmHg
- Molecular Weight 318.329
- Flash Point 206.5 °C
- Transport Information UN 1993
- Appearance White to light yellow crystal powder
- Safety 45-36/37-33-24-16-7-36-26
- Risk Codes 40-22-10-36/38-23/25-11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, T,
T, F,
F, Xi
Xi
- Synonyms Phenolphthalein(8Cl);3,3-Bis(4-hydroxyphenyl)-1(3H)-isobenzofuranone;3,3-Bis(4-hydroxyphenyl)phthalide;3,3-Bis(p-hydroxyphenyl)phthalide;Euchessina;Koprol;Laxogen;Lilo;NSC 10464;NSC 215214;Phthalimetten;Phthalin;Purga;Purgen;Purgophen;Spulmako-lax;
- PSA 66.76000
- LogP 3.56010
Synthetic route

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 90℃; for 5h; | 94% |
| With Noccaea caerulescens extract at 80℃; for 0.25h; | 90% |
| With mixed metal catalyst derived from Thlaspi at 80℃; for 0.0833333h; Friedel-Crafts Acylation; | 90% |
-
-
85-44-9
phthalic anhydride

-
-
108-95-2
phenol

-
A
-
77-09-8
phenolphthalein

-
B
-
596-24-7
spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one

| Conditions | Yield |
|---|---|
| With sulfuric acid at 115 - 120℃; |


| Conditions | Yield |
|---|---|
| With potassium nitrite saure Loesung; |

| Conditions | Yield |
|---|---|
| With tin(IV) chloride | |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| at 180℃; |
-
-
84755-69-1
phenolphthaleine glucuronide

-
-
77-09-8
phenolphthalein

| Conditions | Yield |
|---|---|
| With water at 37℃; for 0.75h; β-glucuronidase; addition of Mn2+, V5+, Ni2+, Co2+, Cu+, Ca2+, Cd2+, or Zn2+; influence of regucalcin; effect of dithiothreitol or dipicolinate; |

-
-
77-09-8
phenolphthalein

| Conditions | Yield |
|---|---|
| With ammonia In chloroform |
-
-
62625-15-4
phenolphthalein dibutyrate

-
-
77-09-8
phenolphthalein

| Conditions | Yield |
|---|---|
| With recombinant Arthrobacter globiformis carboxylesterase; water at 45℃; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| at 25℃; pH=10.5; carbonate buffer; |


| Conditions | Yield |
|---|---|
| at 25℃; pH=10.5; carbonate buffer; |

-
-
85-44-9
phthalic anhydride

-
-
95-48-7
ortho-cresol

-
-
108-95-2
phenol

-
A
-
77-09-8
phenolphthalein

-
B
-
854-76-2
[3-(3'-methyl-4'-hydroxyphenyl)-3-(4''-hydroxyphenyl)phthalide]

-
C
-
596-27-0
o-cresolphthalein

| Conditions | Yield |
|---|---|
| Stage #1: ortho-cresol; phenol With zinc(II) chloride In nitrobenzene at 50 - 60℃; Stage #2: phthalic anhydride With zinc(II) chloride In nitrobenzene at 120 - 130℃; for 4h; Overall yield = 34 %; Overall yield = 5.9 g; |

-
-
77-09-8
phenolphthalein

-
-
7440-44-0
pyrographite

-
-
150-76-5
4-methoxy-phenol

-
-
920-46-7
Methacryloyl chloride

-
-
81266-22-0
phenolphthalein Bis(methacrylate)

| Conditions | Yield |
|---|---|
| With hydrogenchloride; magnesium sulfate; triethylamine In dichloromethane; water | 99.86% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; zinc In water for 96h; Heating; | 99% |
| With palladium 10% on activated carbon; W(OTf)6; hydrogen; acetic acid at 50℃; under 760.051 Torr; for 12h; | 94% |
| With palladium on activated carbon; W(OTf)6; hydrogen In acetic acid at 50℃; under 760.051 Torr; for 12h; | 94% |
| Stage #1: phenolphthalein With sodium hydroxide In water for 0.5h; Stage #2: With zinc In water at 20 - 60℃; | 26.3 mg |

| Conditions | Yield |
|---|---|
| Stage #1: aniline With hydrogenchloride In water for 1h; Dean-Stark; Inert atmosphere; Stage #2: phenolphthalein at 154℃; for 22h; Dean-Stark; | 99% |
| With hydrogenchloride In water at 155 - 165℃; for 16h; | 92% |
| Stage #1: phenolphthalein; aniline; hydrogenchloride at 150 - 171℃; for 13 - 46h; Stage #2: With sodium hydroxide; water Product distribution / selectivity; | 91% |
-
-
77-09-8
phenolphthalein

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; for 18h; Sealed tube; | 98% |
| Stage #1: phenolphthalein With triethylamine In dichloromethane Stage #2: With potassium fluoride; N,N`-sulfuryldiimidazole; trifluoroacetic acid In water at 20℃; for 18h; | 87% |
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; for 12h; |
-
-
77-09-8
phenolphthalein

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 98% |
-
-
77-09-8
phenolphthalein

-
-
106-96-7
propargyl bromide

-
-
142457-74-7
3,3-bis(4-(prop-2-yn-1-yloxy)phenyl)isobenzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol for 1h; Heating; | 96% |
| Stage #1: phenolphthalein With potassium carbonate In dimethyl sulfoxide for 0.5h; Stage #2: propargyl bromide In dimethyl sulfoxide | 80% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; cis-(2-(1H-imidazol-2-yl)pyridine)-(2-(imidazol-2-yl)pyridine)dioxidovanadate(V) tris(aqua); dihydrogen peroxide; potassium bromide In methanol; water at 20℃; for 0.5h; pH=2 - 3; | 96% |
| With benzyltriphenylphosphonium tribromide; calcium carbonate In methanol; dichloromethane at 20℃; for 1h; | 92% |
| With 1-benzyl-1-aza-4-azoniabicyclo<2.2.2>octane bromide; calcium carbonate In methanol at 20℃; for 1.16667h; | 88% |
| With bromine In ethanol; acetic acid Heating; |

| Conditions | Yield |
|---|---|
| With Sulfate; titanium(IV) oxide In dichloromethane at 20℃; for 0.5h; | 96% |
-
-
31643-49-9
4-Nitrophthalonitrile

-
-
77-09-8
phenolphthalein

-
-
72742-00-8
[4,4'-(4,4'-(3-oxo-1,3-dihydroisobenzofuran-1,1-diyl)bis(4,1-phenylene)bis(oxy))]diphthalo nitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 24h; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 8h; |
-
-
39600-44-7
methyl N-phosphonomethylglycinate

-
-
77-09-8
phenolphthalein

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; hydroxylamine In methanol | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: phenolphthalein With hydroxylamine hydrochloride; sodium hydroxide In water at 80℃; Stage #2: With sulfuric acid In water at 100℃; | 92% |
| With potassium hydroxide; hydroxylamine hydrochloride at 80℃; | 76% |
| Stage #1: phenolphthalein With potassium hydroxide; hydroxylamine hydrochloride In water at 80℃; for 0.0833333h; Stage #2: With acetic acid In ethanol; water Stage #3: With sulfuric acid In water for 2h; Heating / reflux; | 76% |
| Stage #1: phenolphthalein With hydroxylamine hydrochloride; sodium hydroxide In water at 80℃; Stage #2: With sulfuric acid In water at 100℃; |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
77-09-8
phenolphthalein

-
-
369384-87-2
C35H20Cl2N4O4

- polymer; monomer(s): 1,4-dihydroxyanthraquinone; phenolphthalein; 3,3'-{[6-(naphthalen-2-ylamino)-1,3,5-triazine-2,4-diyl]bis(oxy)}dinaphthalene-2-carbonyl chloride
-
polymer; monomer(s): 1,4-dihydroxyanthraquinone; phenolphthalein; 3,3'-{[6-(naphthalen-2-ylamino)-1,3,5-triazine-2,4-diyl]bis(oxy)}dinaphthalene-2-carbonyl chloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Heating; | 90% |


| Conditions | Yield |
|---|---|
| In ethanol; toluene at 80℃; for 5h; Mannich Aminomethylation; | 89% |

-
-
77-09-8
phenolphthalein

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 20℃; | 89% |

| Conditions | Yield |
|---|---|
| at 180℃; for 48h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With iodine at 20℃; for 0.8h; Neat (no solvent); Air atmosphere; | 88% |
-
-
3140-73-6
2-chloro-4,6-dimethoxy-1 ,3,5-triazine

-
-
77-09-8
phenolphthalein

-
-
1432754-89-6
3,3-bis(4-((4,6-dimethoxy-1,3,5-triazin-2-yl)oxy)phenyl)isobenzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In tetrahydrofuran at 20℃; | 88% |
-
-
77-09-8
phenolphthalein

-
-
33964-04-4
dinitrophenolphthalein

| Conditions | Yield |
|---|---|
| With yttrium(lll) nitrate hexahydrate In acetic acid at 20℃; for 1h; | 87% |
| With nitric acid In acetic acid at 15 - 20℃; for 6h; Product distribution / selectivity; | 78% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Heating; | 85% |

-
-
77-09-8
phenolphthalein

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
1254084-09-7
3,3-bis(4-hydroxyphenyl)-2-(3-(methylamino)propyl)isoindolin-1-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 24h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| for 24h; Reflux; | 83% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 24h; Reflux; | 82% |

| Conditions | Yield |
|---|---|
| In methanol (inert conditions); stirring soln. of ruthenium compd. and phenolphtalein in methanol at room temp. overnight; evapn., addn. of acetone, storing at -30°C, removal supernatant soln., NMR; | 81% |

| Conditions | Yield |
|---|---|
| In benzyl alcohol heating (150-170°C, 10 h); solvent removal (vac.), pptn. on pouring residue into benzene, filtration, drying (vac.); elem. anal.; | 80% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Heating; | 80% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Heating; | 80% |

-
-
77-09-8
phenolphthalein

-
-
369384-87-2
C35H20Cl2N4O4

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide In N,N-dimethyl-formamide Heating; | 79% |
Phenolphthalein Consensus Reports
Phenolphthalein Specification
The Phenolphthalein with CAS registry number of 77-09-8 is also known as 3,3-Bis(p-hydroxyphenyl)phthalide. The IUPAC name is 3,3-Bis(4-hydroxyphenyl)-2-benzofuran-1-one. It belongs to product categories of Furan&Benzofuran; Analytical Chemistry; Indicator (pH); pH Indicators; Chemistry; Indicator Solutions; Indicators; Titration; Indicator SolutionsStains and Dyes; P; Stains&Dyes, A to. Its EINECS registry number is 201-004-7. In addition, the formula is C20H14O4 and the molecular weight is 318.32. This chemical is a white to light yellow crystal powder and should be stored in sealed containers.
Physical properties about Phenolphthalein are: (1)ACD/LogP: 2.63; (2)ACD/LogD (pH 5.5): 2.63; (3)ACD/LogD (pH 7.4): 2.62; (4)ACD/BCF (pH 5.5): 58.76; (5)ACD/BCF (pH 7.4): 57.9; (6)ACD/KOC (pH 5.5): 642.52; (7)ACD/KOC (pH 7.4): 633.18; (8)#H bond acceptors: 4; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.693; (12)Molar Refractivity: 88.1 cm3; (13)Molar Volume: 229.7 cm3; (14)Surface Tension: 65 dyne/cm; (15)Density: 1.385 g/cm3; (16)Flash Point: 206.5 °C; (17)Enthalpy of Vaporization: 87.1 kJ/mol; (18)Boiling Point: 557.8 °C at 760 mmHg; (19)Vapour Pressure: 4.76E-13 mmHg at 25 °C.
Preparation of Phenolphthalein: it is prepared by condensation of phthalic anhydride with two equivalents of phenol under acidic conditions.
.png)
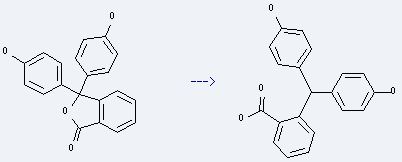
Uses of Phenolphthalein: it is used to produce 2-(4,4'-dihydroxy-benzhydryl)-benzoic acid. The reaction occurs with reagent zinc, sodium hydroxide and solvent H2O with other condition of heating for 96 hours. The yield is about 99%. Besides, it is used to perform a presumptive blood test known as the Kastle-Meyer test. It also is used as an acid or base indicator and used in toys.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. It is toxic by inhalation and if swallowed. However, there is limited evidence of a carcinogenic effect. What's more, it is highly flammable. During using it, wear suitable protective clothing and gloves. Avoid contact with skin. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately and take precautionary measures against static discharges. After using it, keep container tightly closed away from sources of ignition.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C2C(=C1)C(=O)OC2(C3=CC=C(C=C3)O)C4=CC=C(C=C4)O
2. InChI: InChI=1S/C20H14O4/c21-15-9-5-13(6-10-15)20(14-7-11-16(22)12-8-14)18-4-2-1-3-17(18)19(23)24-20/h1-12,21-22H
3. InChIKey: KJFMBFZCATUALV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | TDLo | oral | 29mg/kg (29mg/kg) | GASTROINTESTINAL: GASTRITIS GASTROINTESTINAL: NAUSEA OR VOMITING KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION | Postgraduate Medical Journal. Vol. 60, Pg. 491, 1984. |
| rat | LD | oral | > 1gm/kg (1000mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 25, 1953. | |
| rat | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 30, 1953. |
Related Products
- Phenolphthalein
- Phenolphthalein
- Phenolphthalein dibutyrate
- Phenolphthalein diphosphate
- Phenolphthalein diphosphate calcium salt
- Phenolphthalein diphosphate pyridine salt
- Phenolphthalein diphosphate tetrasodium salt
- Phenolphthalein disulfate tripotassium salt trihydrate
- Phenolphthalein monophosphate dicyclohexylammonium salt
- Phenolphthalein monophosphate disodium salt
- 77098-09-0
- 77102-82-0
- 77102-93-3
- 77106-50-4
- 77107-46-1
- 77108-40-8
- 77112-52-8
- 77112-53-9
- 77112-66-4
- 77113-84-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View