-
Name
Phenylbutazone
- EINECS 200-029-0
- CAS No. 50-33-9
- Article Data30
- CAS DataBase
- Density 1.173 g/cm3
- Solubility water: <0.1 g/100 mL at 23.5 °C
- Melting Point 104-107 °C
- Formula C19H20N2O2
- Boiling Point 424.9 °C at 760 mmHg
- Molecular Weight 308.38
- Flash Point 174.3 °C
- Transport Information
- Appearance off-white powder
- Safety 36/37/39-26-45-22-53
- Risk Codes 36/37/38-20/21/22-42/43-45
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, T
T
- Synonyms 1,2-Diphenyl-3,5-dioxo-4-butylpyrazolidine;1,2-Diphenyl-3,5-dioxo-4-n-butylpyrazolidine;1,2-Diphenyl-4-butyl-3,5-pyrazolidinedione;3,5-Dioxo-1,2-diphenyl-4-n-butylpyrazolidine;
- PSA 40.62000
- LogP 3.91780
Synthetic route
-
-
109-65-9
1-bromo-butane

-
-
2652-77-9
1,2-diphenylpyrazolidine-3,5-dione

-
A
-
50-33-9
Phenylbutazone

-
B
-
102886-57-7
4,4-dibutyl-1,2-diphenylpyrazolidine-3,5-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In hexane; acetonitrile at 55℃; for 18h; Inert atmosphere; Cooling with ice; | A 85% B 15% |


| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In hexane; acetonitrile at 55℃; for 22h; Inert atmosphere; Cooling with ice; | 78% |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| Stage #1: diethyl butylmalonate With sodium ethanolate In ethanol at 20℃; Inert atmosphere; Schlenk technique; Stage #2: diphenyl hydrazine In ethanol for 24h; Reflux; Inert atmosphere; Schlenk technique; | 75% |
| With sodium ethanolate | |
| With sodium ethanolate |

| Conditions | Yield |
|---|---|
| With nickel; benzene |

| Conditions | Yield |
|---|---|
| With sodium ethanolate |

| Conditions | Yield |
|---|---|
| With diethyl ether; diethylamine |

| Conditions | Yield |
|---|---|
| With pyridine; ethanol |

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| With sodium hydride; benzene |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether; bromine / Behandeln einer Loesung des Reaktionsprodukts in Aether mit Phosphor(V)-chlorid 2: diethyl ether; diethylamine View Scheme |
-
-
1229645-19-5
C20H23N2O6P

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / ethanol; water 2: human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride / 37 °C / pH 7.4 / aq. buffer; Enzymatic reaction View Scheme |

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| With human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride at 37℃; pH=7.4; Kinetics; pH-value; Temperature; Reagent/catalyst; aq. buffer; Enzymatic reaction; |
-
-
1229521-07-6
4-butyl-4-((methylthiomethoxy)methyl)-1,2-diphenylpyrazolidine-3,5-dione

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: N-iodo-succinimide / tetrahydrofuran / 12 h / 20 °C 2: palladium 10% on activated carbon; hydrogen / ethanol / 6 h / 20 °C 3: sodium hydroxide / ethanol; water 4: human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride / 37 °C / pH 7.4 / aq. buffer; Enzymatic reaction View Scheme |
-
-
1229521-08-7
dibenzyl ((4-butyl-3,5-dioxo-1,2-diphenylpyrazolidin-4-yl)methoxy)methyl phosphate

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: palladium 10% on activated carbon; hydrogen / ethanol / 6 h / 20 °C 2: sodium hydroxide / ethanol; water 3: human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride / 37 °C / pH 7.4 / aq. buffer; Enzymatic reaction View Scheme |
-
-
1229645-20-8
C21H25N2O7P

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / ethanol; water 2: human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride / 37 °C / pH 7.4 / aq. buffer; Enzymatic reaction View Scheme |

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| With human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride at 37℃; pH=7.4; Kinetics; pH-value; Reagent/catalyst; Temperature; aq. buffer; Enzymatic reaction; |
-
-
23111-33-3
4-n-Butyl-4-hydroxymethyl-1,2-diphenyl-3,5-dioxopyrazolidin

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: acetic anhydride; acetic acid / 24 h / 20 °C / Inert atmosphere 2: N-iodo-succinimide / tetrahydrofuran / 12 h / 20 °C 3: palladium 10% on activated carbon; hydrogen / ethanol / 6 h / 20 °C 4: sodium hydroxide / ethanol; water 5: human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride / 37 °C / pH 7.4 / aq. buffer; Enzymatic reaction View Scheme | |
| Multi-step reaction with 3 steps 1.1: pyridine / dichloromethane / 0.5 h / Cooling with ice; Inert atmosphere 1.2: 2 h / 20 °C / Inert atmosphere 2.1: sodium hydroxide / ethanol; water 3.1: human placental alkaline phosphatase; magnesium chloride; zinc(II) chloride / 37 °C / pH 7.4 / aq. buffer; Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydride / chlorobenzene; mineral oil / 5 h / 0 °C / Reflux 2: potassium carbonate; sodium iodide / acetonitrile; hexane / 22 h / 55 °C / Inert atmosphere; Cooling with ice View Scheme |
-
-
50-33-9
Phenylbutazone

-
-
16860-43-8
4-Butyl-4-hydroxy-1,2-diphenyl-1H-pyrazol-3,5(2H,4H)-dion

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium hydrogencarbonate In water; acetonitrile | 100% |
| With 1H-imidazole; Mn(TDCPP)Cl; dihydrogen peroxide In dichloromethane; acetonitrile at 20℃; for 4h; | 90% |
| With 3-chloro-benzenecarboperoxoic acid In ethanol for 12h; Heating; | 74% |
-
-
50-33-9
Phenylbutazone

-
-
33053-06-4
phenylbutazone hydroperoxide

| Conditions | Yield |
|---|---|
| With air; manganese triacetate In acetic acid at 23℃; for 2h; | 99% |
| With oxygen; manganese triacetate; acetic acid at 23℃; for 2h; | 88% |
| With air; di-tert-butyl peroxide In benzene for 336h; Ambient temperature; | 50% |
-
-
13755-29-8
sodium tetrafluoroborate

-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| Stage #1: Phenylbutazone With trichlorophosphate In toluene for 4h; Reflux; Stage #2: sodium tetrafluoroborate In water | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: C18H14N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 6h; Michael Addition; Inert atmosphere; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-bromophenyl)-1-(1-methyl-1H-imidazol-2-yl)prop-2-en-1-one With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 8h; Michael Addition; Inert atmosphere; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-cinnamoyl-1-methyl-1H-imidazole With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 6h; Reagent/catalyst; Michael Addition; Inert atmosphere; enantioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: C15H16N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 5.5h; Michael Addition; Inert atmosphere; enantioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: C14H14N2O2 With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 5h; Michael Addition; Inert atmosphere; enantioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: C11H10N2OS With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 12h; Michael Addition; Inert atmosphere; enantioselective reaction; | 96% |
-
-
50-33-9
Phenylbutazone

-
-
104-94-9
4-methoxy-aniline

-
-
76383-80-7
2-(N,N'-Diphenyl-hydrazinocarbonyl)-hexanoic acid (4-methoxy-phenyl)-amide

| Conditions | Yield |
|---|---|
| at 150℃; for 5h; | 95% |
-
-
50-33-9
Phenylbutazone

-
-
141293-06-3
1,2-diphenyl-4-n-butyl-4-fluoropyrazolidine-3,5-dione

| Conditions | Yield |
|---|---|
| With N-fluorobis<(trifluoromethyl)sulfonyl>imide In acetic acid for 0.5h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: C14H14N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 7h; Michael Addition; Inert atmosphere; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: C13H9F3N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 5h; Michael Addition; Inert atmosphere; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: C15H14N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 16h; Michael Addition; Inert atmosphere; enantioselective reaction; | 95% |
-
-
90-46-0
9-hydroxyxanthene

-
-
50-33-9
Phenylbutazone

-
-
103325-80-0
1,2-Diphenyl-4-n-butyl-4-xanthen-9-yl-pyrazolidin-3,5-dion

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 0.333333h; Ambient temperature; | 93% |
-
-
50-33-9
Phenylbutazone

-
-
106-49-0
p-toluidine

-
-
76383-78-3
2-(N,N'-Diphenyl-hydrazinocarbonyl)-hexanoic acid p-tolylamide

| Conditions | Yield |
|---|---|
| at 150℃; for 5h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: C13H11ClN2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 8h; Michael Addition; Inert atmosphere; enantioselective reaction; | 93% |
-
-
50-33-9
Phenylbutazone

| Conditions | Yield |
|---|---|
| With palladium diacetate; acetic acid In acetonitrile at 120℃; for 4h; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: C14H14N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 6h; Michael Addition; Inert atmosphere; enantioselective reaction; | 92% |
-
-
50-33-9
Phenylbutazone

-
-
100-46-9
benzylamine

-
-
76383-73-8
2-(N,N'-Diphenyl-hydrazinocarbonyl)-hexanoic acid benzylamide

| Conditions | Yield |
|---|---|
| for 5h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-<3-(4-methylphenyl)acryloyl>-1-methyl-1H-imidazole With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 5h; Michael Addition; Inert atmosphere; enantioselective reaction; | 90% |
-
-
50-33-9
Phenylbutazone

-
-
79202-01-0
3,3'-dithio-bis(4-butyl-1,2-diphenyl-5-thioxo-3-pyrazoline)

| Conditions | Yield |
|---|---|
| With Lawessons reagent In toluene for 1.5h; Heating; | 89% |
| With 2,4-bis(4-methoxyphenyl)-1,3,4,2-dithiadiphosphetane In benzene at 80℃; for 3h; | 85% |

| Conditions | Yield |
|---|---|
| Stage #1: C17H14N2O With C38H38N4O2Rh(1+)*F6P(1-) In 1,2-dichloro-ethane at 23℃; for 0.0833333h; Michael Addition; Schlenk technique; Stage #2: Phenylbutazone In 1,2-dichloro-ethane at 23℃; for 10h; Michael Addition; Inert atmosphere; enantioselective reaction; | 89% |
-
-
50-00-0
formaldehyd

-
-
50-33-9
Phenylbutazone

-
-
23111-33-3
4-n-Butyl-4-hydroxymethyl-1,2-diphenyl-3,5-dioxopyrazolidin

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol; water at 20℃; for 24h; | 86% |
-
-
50-33-9
Phenylbutazone

-
-
2028-76-4
3-nitrophenyldiazonium chloride

-
-
76383-48-7
4-Butyl-4-(3-nitro-phenylazo)-1,2-diphenyl-pyrazolidine-3,5-dione

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 2h; | 86% |
-
-
3315-32-0, 2525-39-5
2,4,6-tri-tert-butylphenoxyl

-
-
50-33-9
Phenylbutazone

-
-
156304-69-7
4-n-Butyl-4-(1,3,5-tri-tert-butyl-4-oxo-cyclohexa-2,5-dien-1-yl)-1,2-diphenyl-pyrazolidin-3,5-dion

| Conditions | Yield |
|---|---|
| In benzene at 25℃; for 6h; | 85% |

| Conditions | Yield |
|---|---|
| With oxygen; potassium iodide; sodium hydroxide In acetonitrile at 80℃; under 760.051 Torr; for 24h; Green chemistry; | 83% |
-
-
50-33-9
Phenylbutazone

-
-
109-05-7, 3000-79-1
2-Methylpiperidin

-
-
76383-74-9
2-(2-Methyl-piperidine-1-carbonyl)-hexanoic acid N,N'-diphenyl-hydrazide

| Conditions | Yield |
|---|---|
| at 150℃; for 5h; | 81% |
-
-
50-33-9
Phenylbutazone

-
-
115262-01-6
[bromo(difluoro)methyl](trimethyl)silane

| Conditions | Yield |
|---|---|
| Stage #1: Phenylbutazone With lithium iodide; sodium t-butanolate In dichloromethane at 20℃; for 0.166667h; Schlenk technique; Glovebox; Inert atmosphere; Stage #2: [bromo(difluoro)methyl]-trimethyl-silane In dichloromethane at 20℃; for 0.5h; Temperature; Schlenk technique; Glovebox; Inert atmosphere; regioselective reaction; | A n/a B 81% |

Phenylbutazone Chemical Properties
IUPAC Name: 4-butyl-1,2-diphenylpyrazolidine-3,5-dione
Empirical Formula: C19H20N2O2
Molecular Weight: 308.3743g/mol
EINECS: 200-029-0
Structure of 4-Butyl-1,2-diphenyl-3,5-dioxopyrazolidine (CAS NO.50-33-9):
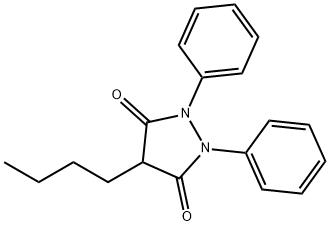
Index of Refraction: 1.59
Molar Refractivity: 88.7 cm3
Molar Volume: 262.7 cm3
Polarizability: 35.16×10-24cm3
Surface Tension: 47.3 dyne/cm
Density: 1.173 g/cm3
Flash Point: 174.3 °C
Enthalpy of Vaporization: 67.94 kJ/mol
Melting Point: 104-107 °C
Boiling Point: 424.9 °C at 760 mmHg
Vapour Pressure: 2E-07 mmHg at 25°C
Water Solubility: <0.1 g/100 mL at 23.5 ºC
Stability: Stable. Incompatible with strong oxidizing agents, strong acids, strong bases.
Product Categories: Intermediates & Fine Chemicals;Pharmaceuticals
Canonical SMILES: CCCCC1C(=O)N(N(C1=O)C2=CC=CC=C2)C3=CC=CC=C3
InChI: InChI=1S/C19H20N2O2/c1-2-3-14-17-18(22)20(15-10-6-4-7-11-15)21(19(17)23)16-12-8-5-9-13-16/h4-13,17H,2-3,14H2,1H3
InChIKey: VYMDGNCVAMGZFE-UHFFFAOYSA-N
Phenylbutazone Uses
4-Butyl-1,2-diphenyl-3,5-dioxopyrazolidine (50-33-9) is a non-steroidal anti-inflammatory compound. An inhibitor of cyclooxygenase that is also a substrate for peroxidation by cyclooxygenase.
Phenylbutazone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | intravenous | 100mg/kg (100mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 36, 1969. | |
| dog | LD50 | intravenous | 121mg/kg (121mg/kg) | BEHAVIORAL: ANALGESIA | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 149, Pg. 571, 1964. |
| dog | LD50 | oral | 332mg/kg (332mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Oyo Yakuri. Pharmacometrics. Vol. 20, Pg. 265, 1980. |
| guinea pig | LD50 | oral | 250mg/kg (250mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 1207, 1969. | |
| hamster | LD50 | oral | 1260mg/kg (1260mg/kg) | Archives of Toxicology, Supplement. Vol. 7, Pg. 365, 1984. | |
| mammal (species unspecified) | LD50 | intraperitoneal | 300mg/kg (300mg/kg) | BEHAVIORAL: ANALGESIA | European Journal of Medicinal Chemistry--Chimie Therapeutique. Vol. 21, Pg. 277, 1986. |
| mammal (species unspecified) | LD50 | oral | 700mg/kg (700mg/kg) | BEHAVIORAL: ANALGESIA | European Journal of Medicinal Chemistry--Chimie Therapeutique. Vol. 21, Pg. 277, 1986. |
| man | TDLo | oral | 17500ug/kg/3W (17.5mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED | Annals of Internal Medicine. Vol. 41, Pg. 1075, 1954. |
| man | TDLo | oral | 2426mg/kg/24H (2426mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Annals of Emergency Medicine. Vol. 20, Pg. 204, 1991. |
| man | TDLo | unreported | 200mg/kg/5W-I (200mg/kg) | VASCULAR: BP ELEVATION NOT CHARACTERIZED IN AUTONOMIC SECTION KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED KIDNEY, URETER, AND BLADDER: HEMATURIA | British Medical Journal. Vol. 282, Pg. 950, 1981. |
| mouse | LD50 | intramuscular | 430mg/kg (430mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Oyo Yakuri. Pharmacometrics. Vol. 13, Pg. 97, 1977. |
| mouse | LD50 | intraperitoneal | 128mg/kg (128mg/kg) | Pharmaceutical Chemistry Journal Vol. 19, Pg. 33, 1985. | |
| mouse | LD50 | intravenous | 90mg/kg (90mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 36, 1969. | |
| mouse | LD50 | oral | 238mg/kg (238mg/kg) | Pharmacological Research Communications. Vol. 18, Pg. 241, 1986. | |
| mouse | LD50 | subcutaneous | 230mg/kg (230mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 7, Pg. 1022, 1955. | |
| mouse | LD50 | unreported | 128mg/kg (128mg/kg) | Farmatsevtichnii Zhurnal Vol. (5), Pg. 26, 1983. | |
| rabbit | LD50 | intravenous | 146mg/kg (146mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 129, 1960. |
| rabbit | LD50 | oral | 781mg/kg (781mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Oyo Yakuri. Pharmacometrics. Vol. 20, Pg. 265, 1980. |
| rat | LD50 | intramuscular | 220mg/kg (220mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 665, 1960. | |
| rat | LD50 | intraperitoneal | 142mg/kg (142mg/kg) | Farmaco, Edizione Scientifica. Vol. 14, Pg. 347, 1959. | |
| rat | LD50 | intravenous | 100mg/kg (100mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 665, 1960. | |
| rat | LD50 | oral | 245mg/kg (245mg/kg) | BEHAVIORAL: ANALGESIA | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 123, Pg. 48, 1959. |
| rat | LD50 | subcutaneous | 230mg/kg (230mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: TREMOR | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 1285, 1972. |
| women | TDLo | oral | 168mg/kg/4W-I (168mg/kg) | VASCULAR: OTHER CHANGES KIDNEY, URETER, AND BLADDER: CHANGES PRIMARILY IN GLOMERULI | Archives of Internal Medicine. Vol. 145, Pg. 685, 1985. |
| women | TDLo | unreported | 40mg/kg/4D-I (40mg/kg) | BLOOD: LEUKOPENIA BLOOD: AGRANULOCYTOSIS | Problemy Gematologii i Perelivaniia Krovi. Problems of Hematology and Blood Transfusion. Vol. 4(5), Pg. 48, 1959. |
Phenylbutazone Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 316.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 13 (1977),p. 183.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
Phenylbutazone Safety Profile
Hazard Codes:  Xn,
Xn, T
T
Risk Statements: 36/37/38-20/21/22-42/43-45
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R42/43:May cause sensitization by inhalation and skin contact.
R45:May cause cancer.
Safety Statements: 36/37/39-26-45-22-53
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S22:Do not breathe dust.
S53:Avoid exposure - obtain special instructions before use.
RIDADR: 3249
RTECS: UQ8225000
HazardClass: 6.1(b)
PackingGroup: III
Hazardous Substances Data: 50-33-9(Hazardous Substances Data)
Suspected human carcinogen producing leukemia. A human poison by parenteral route. An experimental poison by ingestion, intraperitoneal, subcutaneous, intravenous, and intramuscular routes. Human systemic effects by ingestion and possibly other routes: fever, blood pressure increase, other unspecified vascular effects, damage to kidney tubules and glomeruli, decreased urine volume, blood in the urine, reduction in the number of white blood cells, and agranulocytosis. Experimental teratogenic and reproductive effects. Human mutation data reported. An eye irritant. An anti-inflammatory agent. When heated to decomposition it emits toxic fumes of NOx.
Phenylbutazone Specification
4-Butyl-1,2-diphenyl-3,5-dioxopyrazolidine , its cas register number is 50-33-9. It also can be called 'Esteve' ; 1,2-Diphenyl-3,5-dioxo-4-butylpyrazolidine ; 3,5-Dioxe-4 buty-1, diphenyl-pyrazolidine ; 5-24-05-00400 (Beilstein Handbook Reference) ; Alindor ; Alkabutazona ; Alqoverin ; Anerval ; Anpuzone ; Antadol ; Anuspiramin ; Arthrizon ; Artrizin ;
Artrizone ; Artropan ; Azobutyl ; Azolid ; Benzone ; Betazed ; Bizolin ; Bizolin 200 ; Bunetzone ; Busone ; Buta phen ;
Butacompren ; Butacote ; Butadion ; Butadiona ; Butadione ; Butadionum ; Butagesic ; Butalgina ; Butalidon ; Butaluy ;
Butaphen ; Butapirazol ; Butapirazole ; Butapyrazole ; Butarecbon ; Butartril ; Butartrina ; Butazina ; Butazolidin ;
Butazolidine ; Butazona ; Bute ; Butidiona ; Butiwas-simple ; Butone ; Butoz ; Butylpyrin ; Buvetzone ; Buzon ;
Chembutazone ; Digibutina ; Diossidone ; Diozol ; Diphebuzol ; Diphenylbutazone ; Ecobutazone ; Elmedal ; Equi bute ;
Equipalazone ; Eributazone ; Exrheudon N ; Febuzina ; Fenartil ; Fenibutal ; Fenibutasan ; Fenibutazona ; Fenibutol ;
Fenilbutazona ; Fenilbutazone ; Fenilbutina ; Fenilbutine ; Fenilidina ; Fenotone ; Fenylbutazon ; Intalbut ; Intrabutazone ;
Mephabutazon ; Phebuzin ; Phebuzine ; Phen-Buta-Vet ; Phenbutazol ; Phenopyrine ; Phenyl-mobuzon ; Pyrazolidin ;
Scanbutazone ; Schemergen ; UNII-GN5P7K3T8S . 4-Butyl-1,2-diphenyl-3,5-dioxopyrazolidine (CAS NO.50-33-9) is a off-whtie Solid.
Related Products
- Phenylbutazone
- Phenylbutazone calcium
- 50-34-0
- 503-40-2
- 5034-06-0
- 50340-79-9
- 503417-29-6
- 503417-30-9
- 503417-36-5
- 503417-37-6
- 503417-38-7
- 50342-08-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View