-
Name
CYCLOHEXYLPHOSPHINE
- EINECS
- CAS No. 822-68-4
- Article Data14
- CAS DataBase
- Density 0,875 g/cm3
- Solubility
- Melting Point
- Formula C6H13P
- Boiling Point 145 °C at 760 mmHg
- Molecular Weight 116.143
- Flash Point 41.5 °C
- Transport Information UN 3185
- Appearance colourless liquid with an offensive odour
- Safety 9-16-26-36/37/39
- Risk Codes 17-20/21/22-34
-
Molecular Structure
- Hazard Symbols
- Synonyms Cyclohexylphosphine;
- PSA 13.59000
- LogP 2.19420
Synthetic route
-
-
2310-71-6
dicyclohexylphosphonous acid

-
A
-
1005-23-8
cyclohexylphosphonic acid

-
B
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| at 250℃; Yields of byproduct given; | A n/a B 46.13% |
| With water 1) 250 deg C, 2) reflux; Yield given. Multistep reaction. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 4h; Product distribution; Heating; reaction with KOH; | A n/a B 33% C n/a |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether | |
| With sodium; toluene |

| Conditions | Yield |
|---|---|
| With phosphan Irradiation; | |
| With 2,2'-azobis(isobutyronitrile); phosphan at 78 - 80℃; for 3.5h; |

| Conditions | Yield |
|---|---|
| UV-Licht.Irradiation; |
-
-
80984-31-2
Os3(CO)11P(C6H11)H2

-
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| In toluene 100°C, 24 h; vac. evapd., chromd. on silica gel with 5/1 pentane/toluene; | 60-66 |

| Conditions | Yield |
|---|---|
| Stage #1: dichlorocyclohexylphosphine With sodium In 1,4-dioxane at 110℃; Inert atmosphere; Stage #2: With acetic acid In diethyl ether at -10 - 0℃; for 5h; Inert atmosphere; |
-
-
31080-62-3
diisopropylallylphosphonite

-
-
822-68-4
cyclohexylphosphine

-
-
850716-19-7
C6H11P(C3H6P(OCH(CH3)2)2)2

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) at 20℃; for 48h; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) at 20℃; for 48h; Irradiation; | 100% |
-
-
109-64-8
1,3-dibromo-propane

-
-
822-68-4
cyclohexylphosphine

-
-
97472-13-4
1,3-bis(cyclohexylphosphanyl)propane

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexylphosphine With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.333333h; Inert atmosphere; Stage #2: 1,3-dibromo-propane In tetrahydrofuran; hexane at -78 - 20℃; Inert atmosphere; | 100% |
-
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; acetic acid In tetrahydrofuran at 0 - 20℃; | 99.5% |
-
-
109-04-6
2-bromo-pyridine

-
-
822-68-4
cyclohexylphosphine

-
-
380358-80-5
cyclohexylbis(2-pyridyl)phosphane

| Conditions | Yield |
|---|---|
| With triethylamine; tetrakis(triphenylphosphine) palladium(0) In acetonitrile for 36h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 25℃; | 98% |
| In ethanol at 70℃; for 2h; Inert atmosphere; | 98% |
| In hexane; water; acetonitrile at 20℃; for 0.5h; Inert atmosphere; |

-
-
822-68-4
cyclohexylphosphine

-
-
316803-49-3
Mn2(μ-H)(μ-P(cyclo-C6H11)2)(CO)7(ax-H2P(cyclo-C6H11))

| Conditions | Yield |
|---|---|
| With Me3NO In tetrahydrofuran metal-complex and H2PCy soln. in THF was cooled to 0 °C in ice-bath, 2 h; chromy. on silica 60, eluent CH2Cl2/hexane=1:1, solvent was removed in high-vac.; | 98% |

-
-
822-68-4
cyclohexylphosphine

-
-
191542-23-1
(cyclohexylphosphane)(methylcyclopentadienyl)molybdenium tetrachloride

| Conditions | Yield |
|---|---|
| In toluene (argon); stirring (room temp., 2 d); solvent removal (vac.), washing (hexane), filtration, drying (vac.), addn. of toluene, filtration, pptn. with hexane; elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 80℃; | 97% |
-
-
7550-45-0
titanium tetrachloride

-
-
822-68-4
cyclohexylphosphine

-
-
469886-23-5, 165281-61-8
(TiCl4(C6H11PH2)2)

| Conditions | Yield |
|---|---|
| In hexane; toluene under N2 CyPH2 was added to soln. TiCl4 in hexane-toluene; ppt. was filtered, washed with hexane and dried in vacuo; elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 80℃; | 96% |

-
-
822-68-4
cyclohexylphosphine

-
-
316803-45-9
Re2(μ-H)(μ-P(cyclo-C6H11)2)(CO)7(ax-H2P(cyclo-C6H11))

| Conditions | Yield |
|---|---|
| With Me3NO In tetrahydrofuran metal-complex and H2PCy soln. in THF was cooled to 0 °C in ice-bath, 2 h; chromy. on silica 60, eluent CH2Cl2/hexane=1:1, solvent was removed in high-vac.; | 95% |
-
-
80432-35-5
pentamethylcyclopentadienyl niobium(V) tetrachloride

-
-
822-68-4
cyclohexylphosphine

-
-
499219-56-6
C5(CH3)5NbCl4(PH2C6H11)

| Conditions | Yield |
|---|---|
| In toluene in dry Ar atmosphere C5(CH3)5NbCl4 suspended in toluene, equimolar amt. of PH2C6H11 added, stirred at room temp. until clear soln. obtained; solvent removed to dryness, residue extd. with-pentane, cooled to 5 °C, crystn.; elem. anal.; | 95% |

-
-
822-68-4
cyclohexylphosphine

-
-
406943-00-8
C5(CH3)5TaCl4(PH2C6H11)

| Conditions | Yield |
|---|---|
| In toluene in dry Ar atmosphere C5(CH3)5TaCl4 suspended in toluene, equimolar amt. of PH2C6H11 added, stirred at room temp. until clear soln. obtained; solvent removed to dryness, residue extd. with-pentane, cooled to 5 °C, crystn.; elem. anal.; | 95% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 80℃; | 94% |
-
-
1121-53-5
benzyl sodium

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
7289-92-1
tris(dimethylamino)stibine

-
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane; toluene under Ar; Sb(NMe2)3 in toluene added to chilled soln. of C6H11PH2 (1:1)in hexane; warmed to room temp.; stirred for 10 min; added to soln. prepared from PhCH2Na and CyPH2 in hexane-THF at -20°C; excess TMEDAadded; warmed to 25°C; reflux; filtered while warm; stored at -35°C; | 93% |

-
-
850794-17-1
[CyP(CH2SiMe2NPh)2]TaMe3

-
-
822-68-4
cyclohexylphosphine

-
-
850794-23-9
[((CyP(CH2SiMe2NPh)2)Ta)2(μ-H)2(μ-PCy)]

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: H2; (Ar); Schlenk or glovebox technique; phosphine was added via microsyringe to soln. of Ta complex in Et2O; stirred for 30 min; solvent removed (vac.); dissolved in pentane; dried (vac.); elem. anal.; | 93% |

| Conditions | Yield |
|---|---|
| In toluene (N2), Schlenk technique; addn. of (C6H11)PH2 to soln. of B(C6F5)3 in toluene, stirring for 1 h at 25°C; evapn. in vacuo; | 93% |
| In toluene at 25℃; Inert atmosphere; | |
| In toluene phosphine combined with B(C6F5)3 in toluene; stirred at 25°C for 1 h; solvent concd.; detd. by (1)H, (11)B, (13)C, (19)F and (31)P NMR spectra; |
-
-
165550-99-2
ReMn(μ-H)(μ-P(cyclo-C6H11)2)(CO)8

-
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| With Me3NO In tetrahydrofuran metal-complex and H2PCy soln. in THF was cooled to 0 °C in ice-bath, 2 h; chromy. on silica 60, eluent CH2Cl2/hexane=1:1, solvent was removed in high-vac.; | 92% |
-
-
697299-24-4
[(PhB(CH2P(i-Pr2)3)Ir(H)(η3-C8H13)]

-
-
822-68-4
cyclohexylphosphine

-
-
697299-29-9
[(C6H5B(CH2P(CH(CH3)2)2)3)IrH2(PH2(C6H11))]

| Conditions | Yield |
|---|---|
| In benzene byproducts: 1,3-cyclooctadiene; (N2); Schlenk technique; neat PH2(C6H11) (1 equiv.) was added to soln. of Ir complex in C6H6 at room temp.; soln. was heated at 65°C for 14 h; filtered through Celite; solvent removed (vac.); elem. anal.; | 92% |
-
-
138695-27-9
(CO)3{P(CH3)3}2Re(OSO2CF3)

-
-
822-68-4
cyclohexylphosphine

-
-
144346-05-4
(CO)3{P(CH3)3}2Re{PH2(C6H11)}(1+)*OSO2CF3(1-)={(CO)3(P(CH3)3)2Re(PH2(C6H11))}(O3SCF3)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. THF (syringe) to evacuated Schlenk tube contg. Re-complex (previously flushed with Ar 3 times), mixt. stirred under Ar until complex dissolved, addn. phosphine (syringe), heated under reflux condenser overnight at 45°C; removal volatile materials (vac.), white residue washed twice (pentane), residue dissolved in THF, concd., layered with Et2O, cooled (-40°C, 2d), crystn., supernatant liq. removed (cannula), washed (pentane), dried (high vac., 12h), elem. anal.; | 91% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: neopentane; Ar-atmosphere; 50°C (40 h); evapn., extg. (C6H6); elem. anal.; | 90.9% |

| Conditions | Yield |
|---|---|
| With Mg In tetrahydrofuran N2-atmosphere; stirring of Zr-complex and excess of Mg (0.5 h), filtration, addn. of CyPH2; crystn. (overnight); | 90% |
| With n-BuLi In tetrahydrofuran N2-atmosphere; addn. of BuLi to Zr-complex (-78°C), warming to room temp. (during 1 h), cooling (-78°C), addn. of CyPH2; crystn. (overnight, 25°C); elem. anal.; | 50% |

-
-
822-68-4
cyclohexylphosphine

-
-
869802-98-2
[Fe2(η5-C5H5)2(μ-CO)2(CO)(CyPH2)]

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); std. Schlenk technique; mixt. of Fe complex and P ligand in CH2Cl2was stirred at room temp. for 5 min; filtered; evapd. (vac.); washed (petroleum ether); elem. anal.; | 90% |

-
-
822-68-4
cyclohexylphosphine

-
-
850794-22-8
[((PhP(CH2SiMe2NPh)2)Ta)2(μ-H)2(μ-PCy)]

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: H2; (Ar); Schlenk or glovebox technique; phosphine was added via microsyringe to soln. of Ta complex in Et2O; stirred for 30 min; solvent removed (vac.); dissolved in pentane; dried (vac.); | 89% |

-
-
822-68-4
cyclohexylphosphine

-
-
937801-08-6
(N(Me3SiNCH2CH2)3)ZrPHCy

| Conditions | Yield |
|---|---|
| In benzene (N2); soln. of cyclohexylphosphine in benzene was added to soln. of zirconium complex in benzene, stirred for 10 min; soln. was frozen, lyophilized; | 89% |
-
-
487-68-3
mesytaldehyde

-
-
822-68-4
cyclohexylphosphine

-
-
1246895-42-0
cyclohexyl-(2,4,6-trimethylbenzyl)phosphine oxide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 80℃; | 89% |
-
-
10026-12-7
niobium pentachloride

-
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| In dichloromethane Ar-atmosphere; addn. of phosphine to slight excess of NbCl5 at -78°C, stirring at -78°C for 1 h, then at room temp. for 13 h; filtration (Celite), removal of volatiles (reduced pressure); elem. anal.; | 88% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 80℃; | 88% |
-
-
10026-12-7
niobium pentachloride

-
-
822-68-4
cyclohexylphosphine

| Conditions | Yield |
|---|---|
| In dichloromethane Ar-atmosphere; slight excess of phosphine, stirring at room temp. for 18h; filtration off of phosphonium salt, crystn. on layering with Et2O (24 h), decantation, drying (vac.); elem. anal.; | A 85% B 68% |
| In dichloromethane-d2 Ar-atmosphere; 1.9 equiv. phosphine, stirring at room temp. for 18 h; filtration off of phosphonium salt, crystn. on cooling filtrate to -20°C for 24 h, solvent removal; IR and NMR spectroscopy; | A 28% B 40% |

Phosphine, cyclohexyl- Specification
This chemical is called Phosphine, cyclohexyl-, and its CAS registry number is 822-68-4. With the molecular formula of C6H13P, its product category is Primary Phosphines. It's incompatible with organic material, air, oxidizing agents, halogens. Store it under an inert atmosphere.
Other characteristics of the Phosphine, cyclohexyl- can be summarised as followings: (1)#H bond acceptors: 0; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 1; (4)Polar Surface Area: 13.59 Å2; (5)Flash Point: 41.5 °C; (6)Enthalpy of Vaporization: 36.63 kJ/mol; (7)Boiling Point: 145 °C at 760 mmHg; (8)Vapour Pressure: 6.25 mmHg at 25°C.
Uses of this chemical: The Phosphine, cyclohexyl- could react with 3-chlorocarbonyloxy-propene, and obtain the Monoallyloxycarbonyl-cyclohexylphosphan. This reaction needs the reagent of K2CO3, and the solvent of benzene. The yield is 73.7 %. In addition, this reaction should be taken for 6 hours. The other condition is heating.
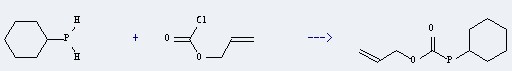
When you are using this chemical, please be cautious about it as the following: This chemical is spontaneously flammable in air. Keep it away from sources of ignition. It's harmful by inhalation, in contacting with skin and if swallowed. It causes burns. Wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, rinse immediately with plenty of water and seek medical advice. Keep the container in a well-ventilated place.
You can still convert the following datas into molecular structure:
1.SMILES: PC1CCCCC1
2.InChI: InChI=1/C6H13P/c7-6-4-2-1-3-5-6/h6H,1-5,7H2
3.InChIKey: ZBCKWHYWPLHBOK-UHFFFAOYAG
Related Products
- Phosphine
- Phosphine oxide,bis(1,1-dimethylethyl)-
- Phosphine oxide,cyclohexyldiphenyl-
- Phosphine oxide,diphenyl-2-propen-1-yl-
- Phosphine oxide,dodecyldimethyl-
- Phosphine oxide,tris(4-methylphenyl)-
- Phosphine selenide,tris(4-methylphenyl)-
- Phosphine sulfide,triethyl-
- Phosphine sulfide,trimethyl- (6CI,7CI,8CI,9CI)
- Phosphine, cyclohexyl-
- 82278-73-7
- 82278-95-3
- 82-27-9
- 82279-70-7
- 82-28-0
- 822-82-2
- 822-85-5
- 822-86-6
- 822-87-7
- 822-89-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View