-
Name
(R)-(+)-1,2-Dithiolane-3-pentanoic acid
- EINECS 638-752-2
- CAS No. 1200-22-2
- Article Data52
- CAS DataBase
- Density 1.218 g/cm3
- Solubility
- Melting Point 48-52 °C(lit.)
- Formula C8H14O2S2
- Boiling Point 362.5 °C at 760 mmHg
- Molecular Weight 206.33
- Flash Point 173 °C
- Transport Information
- Appearance yellow crystalline solid
- Safety 20-36-26-35
- Risk Codes 22-36/38
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,2-Dithiolane-3-pentanoicacid, (R)-;1,2-Dithiolane-3-valeric acid, (+)- (8CI);1,2-Dithiolane-3-pentanoicacid, (3R)-;(R)-Lipoic acid;(R)-a-Lipoic acid;Berlition;Byodinoral 300;Lipoec;Lipoic acid;R-(+)-Thioctic acid;Thioderm;Thiogamma;Tiobec;Tiobec Retard;d-Thioctic acid;a-(+)-Lipoic acid;a-Lipoic acid;
- PSA 87.90000
- LogP 2.78510
Synthetic route

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (S)-6,8-dichlorooctanoic acid ethylester With sodium sulfide; sulfur In water at 80℃; for 2h; Stage #2: With water; sodium hydroxide at 80℃; for 8h; Temperature; | 99.8% |
| With sodium sulfide; sulfur | |
| Stage #1: (S)-6,8-dichlorooctanoic acid ethylester With sodium sulfide; sulfur In water at 82℃; for 4.5h; Stage #2: With water; sodium hydroxide In toluene at 92℃; for 8h; pH=1; Solvent; Temperature; | n/a |
-
-
97961-65-4
(+)-isopropyl lipoate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In methanol at 22℃; for 40h; | 96% |
-
-
188412-21-7
(R)-6,8-dichloroctanoic acid

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium sulfide; N-benzyl-N,N,N-triethylammonium chloride; sulfur In water at 85℃; Reagent/catalyst; Temperature; | 92.1% |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With citric acid In methanol; toluene at 35 - 40℃; for 3h; Temperature; Darkness; Industrial scale; | 83.1% |
-
-
104726-74-1
[ethyl (5R)-5-(1,2-dithiolan-3yl)pentanoate]

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium hydroxide In ethanol for 24h; Ambient temperature; | 75% |
| With potassium hydroxide In ethanol at 20℃; for 24h; | 75% |
| With potassium hydroxide In ethanol at 20℃; for 24h; | 75% |
| hydrolysis; | |
| With sodium hydroxide |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Product distribution / selectivity; | 72% |
-
-
99427-00-6
(R)-5-[1,2-dithiolan-3-yl]pentanoic acid methyl ester

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 3h; Ambient temperature; | 70% |
| With potassium hydroxide; water for 24h; | 54% |
| Stage #1: (R)-5-[1,2-dithiolan-3-yl]pentanoic acid methyl ester With potassium hydroxide In methanol; water at 50℃; for 2h; Stage #2: With phosphoric acid In methanol; water; toluene at 30℃; | 45% |
| With potassium carbonate In methanol; water |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| 52% |

-
A
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
B
-
62-46-4, 1077-27-6, 1077-28-7, 1200-22-2, 52578-51-5
(S)-lipoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetone at 15 - 60℃; for 2h; Temperature; Solvent; | A 47.5% B n/a |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetone at 15 - 40℃; for 24h; Solvent; | 46% |
-
-
107554-84-7
(S)-6,8-dimethylsulfonyloxyoctane-1-carboxylic acid

-
A
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
B
-
62-46-4, 1077-27-6, 1077-28-7, 1200-22-2, 52578-51-5
(S)-lipoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium sulfide; potassium hydroxide In water; N,N-dimethyl-formamide at 80℃; for 28h; Title compound not separated from byproducts; | A n/a B 45% |

| Conditions | Yield |
|---|---|
| With ammonium hydrogen sulfite; sodium sulfide nonahydrate; sulfur In propan-1-ol; water; isopropyl alcohol at 70℃; for 3.5h; Temperature; Solvent; Reagent/catalyst; Inert atmosphere; Darkness; | 30.6% |
-
-
1077-28-7, 62-46-4
Thioctic acid

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With cinchonidine salt |
-
-
119365-69-4
(R)-6,8-dimercaptooctanoic acid

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With iron(III) chloride; potassium carbonate | |
| With chloroform; iodine; potassium iodide | |
| With mushroom tyrosinase |
-
-
107554-84-7
(S)-6,8-dimethylsulfonyloxyoctane-1-carboxylic acid

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium sulfide; potassium hydroxide; sulfur 1.) 5 min, 2.) DMF, 80 deg C, 24h; Yield given. Multistep reaction; |
-
-
114529-48-5
(7R,10S)-7-Isopropyl-10-methyl-1,5-dithia-spiro[5.5]undecane

-
A
-
3391-87-5
l-menthone

-
B
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
139685-60-2
(3S)-6-bromo-1,3-isopropylidendioxyhexane

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid
-
-
46236-19-5
1,2-dithiolane-3-pentanoic acid methyl ester

-
A
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
B
-
62-46-4, 1077-27-6, 1077-28-7, 1200-22-2, 52578-51-5
(S)-lipoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol Yield given. Title compound not separated from byproducts; |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With ethanol; sodium disulfide |
-
-
116349-04-3
(6S)-(-)-methyl 6,8-dihydroxyoctanoate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Et3N / CH2Cl2 2: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 3: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 4 steps 1: Et3N / CH2Cl2 / 1 h / 0 °C 2: dimethylformamide; cyclohexane / 4 h / 50 °C 3: H2O; KOH / 6 h / 20 °C 4: FeCl3; air; NaOH / H2O / 3 h / pH 9 View Scheme | |
| Multi-step reaction with 3 steps 1: 92 percent / Et3N / CH2Cl2 / 0.5 h / 0 °C 2: 83 percent / Na2S*9H2O, sulfur / dimethylformamide / 2.42 h / 80 °C 3: 70 percent / aq. KOH / methanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: 98 percent / Et3N 2: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 3: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
116315-78-7
(S)-6,8-diphenylmethoxyoctanoic acid

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1.75 g / diethyl ether 2: 87 percent / H2 / Pd/C / ethanol / 5 h / 2585.81 Torr 3: Et3N / CH2Cl2 4: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 5: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 5 steps 1: 3percent HCl 2: 95 percent / H2 / 5percent palladium on charcoal / methanol / 1551.4 Torr 3: 98 percent / Et3N 4: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 5: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
116349-05-4
(6S)-(+)-methyl 6,8-bis(methylsulfonyloxy)octanoate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 2: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 3 steps 1: dimethylformamide; cyclohexane / 4 h / 50 °C 2: H2O; KOH / 6 h / 20 °C 3: FeCl3; air; NaOH / H2O / 3 h / pH 9 View Scheme | |
| Multi-step reaction with 2 steps 1: 83 percent / Na2S*9H2O, sulfur / dimethylformamide / 2.42 h / 80 °C 2: 70 percent / aq. KOH / methanol / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 2: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
116315-75-4
(S)-1-phenylmethoxyoct-7-en-3-ol

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 85 percent / TBAI; NaH / dimethylformamide / 2 h / 20 °C 2.1: BH3*DMS / tetrahydrofuran / 2 h / 20 °C 2.2: 88 percent / aq. NaOEt; H2O2 / tetrahydrofuran / 2 h / 20 °C 3.1: aq. NaCl2O; NaOCl; TEMPO / acetonitrile; aq. phosphate buffer / 35 °C / pH 6.7 4.1: 1.75 g / diethyl ether 5.1: 87 percent / H2 / Pd/C / ethanol / 5 h / 2585.81 Torr 6.1: Et3N / CH2Cl2 7.1: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 8.1: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 8 steps 1: 95 percent 2: 89 percent / diisopentylborane / tetrahydrofuran / 0 °C 3: pyridinium dichromate / dimethylformamide / 0 deg C, then RT, 24 h 4: 3percent HCl 5: 95 percent / H2 / 5percent palladium on charcoal / methanol / 1551.4 Torr 6: 98 percent / Et3N 7: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 8: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
116315-76-5
(S)-6,8-diphenylmethoxyoct-1-ene

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: BH3*DMS / tetrahydrofuran / 2 h / 20 °C 1.2: 88 percent / aq. NaOEt; H2O2 / tetrahydrofuran / 2 h / 20 °C 2.1: aq. NaCl2O; NaOCl; TEMPO / acetonitrile; aq. phosphate buffer / 35 °C / pH 6.7 3.1: 1.75 g / diethyl ether 4.1: 87 percent / H2 / Pd/C / ethanol / 5 h / 2585.81 Torr 5.1: Et3N / CH2Cl2 6.1: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 7.1: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 7 steps 1: 89 percent / diisopentylborane / tetrahydrofuran / 0 °C 2: pyridinium dichromate / dimethylformamide / 0 deg C, then RT, 24 h 3: 3percent HCl 4: 95 percent / H2 / 5percent palladium on charcoal / methanol / 1551.4 Torr 5: 98 percent / Et3N 6: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 7: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
116315-77-6
(S)-6,8-diphenylmethoxyoctan-1-ol

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: aq. NaCl2O; NaOCl; TEMPO / acetonitrile; aq. phosphate buffer / 35 °C / pH 6.7 2: 1.75 g / diethyl ether 3: 87 percent / H2 / Pd/C / ethanol / 5 h / 2585.81 Torr 4: Et3N / CH2Cl2 5: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 6: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 6 steps 1: pyridinium dichromate / dimethylformamide / 0 deg C, then RT, 24 h 2: 3percent HCl 3: 95 percent / H2 / 5percent palladium on charcoal / methanol / 1551.4 Torr 4: 98 percent / Et3N 5: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 6: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
116315-79-8
(S)-methyl 6,8-diphenylmethoxyoctanoate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 87 percent / H2 / Pd/C / ethanol / 5 h / 2585.81 Torr 2: Et3N / CH2Cl2 3: Na2S; sulphur / dimethylformamide / 24 h / 90 °C 4: K2CO3 / methanol; H2O View Scheme | |
| Multi-step reaction with 4 steps 1: 95 percent / H2 / 5percent palladium on charcoal / methanol / 1551.4 Torr 2: 98 percent / Et3N 3: 1.) sodium sulphide nonahydrate, sulphur / 1.) DMF, 80 deg C, 1 h, 2.)DMF, 80 deg C, 67 h, then RT, 17 h 4: 54 percent / 0.1M KOH, H2O / 24 h View Scheme |
-
-
104726-72-9
ethyl (S)-6,8-dihydroxy octanoate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / Et3N / CH2Cl2 / 4 h / 0 °C 2: 72 percent / Na2S; S / dimethylformamide / 24 h / 90 °C 3: 75 percent / KOH / ethanol / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: Et3N / CH2Cl2 / 4 h / 0 °C 2: 70 percent / sodium sulfide nonahydrate, sulfur / dimethylformamide / 24 h / 90 °C 3: 75 percent / 1.) 0.1M potassium hydroxide, 2.) 5M HCl / ethanol / 24 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 2: Na2S - S / dimethylformamide / 90 °C 3: hydrolysis View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 4 h / 0 °C 2: sodiumsulfide nonahydrate; sulfur / N,N-dimethyl-formamide / 24 h / 85 - 90 °C 3: potassium hydroxide / ethanol / 24 h / 20 °C View Scheme |
-
-
104726-73-0
(S)-Ethyl 6,8-Bis(methylsulphonyloxy)octanoate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 72 percent / Na2S; S / dimethylformamide / 24 h / 90 °C 2: 75 percent / KOH / ethanol / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 70 percent / sodium sulfide nonahydrate, sulfur / dimethylformamide / 24 h / 90 °C 2: 75 percent / 1.) 0.1M potassium hydroxide, 2.) 5M HCl / ethanol / 24 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: Na2S - S / dimethylformamide / 90 °C 2: hydrolysis View Scheme | |
| Multi-step reaction with 2 steps 1: sodiumsulfide nonahydrate; sulfur / N,N-dimethyl-formamide / 24 h / 85 - 90 °C 2: potassium hydroxide / ethanol / 24 h / 20 °C View Scheme |
-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
-
119365-69-4
(R)-6,8-dimercaptooctanoic acid

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 1h; | 100% |
| With 1,4-dihydronicotinamide adenine dinucleotide; porcine heart dihydrolipoamide dehydrogenase at 35℃; Enzyme kinetics; | |
| With sodium borohydrid In water; toluene |
-
-
657-24-9
dimethylbiguanide

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
-
959986-37-9
N,N-dimethylimidodicarbonimidic diamide R-(+)-lipoate

| Conditions | Yield |
|---|---|
| In methanol for 2h; Product distribution / selectivity; | 100% |
| In acetonitrile for 0.166667h; Product distribution / selectivity; | 95% |
| In acetone for 0.166667 - 0.5h; Product distribution / selectivity; | 95% |
| In methanol; acetone for 0.333333h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; | 100% |
| In ethanol at 20 - 50℃; for 0.5h; | 68% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium borohydrid; sodium hydrogencarbonate In water | 100% |
| With sodium tetrahydroborate; sodium hydrogencarbonate In water at 5 - 20℃; for 1.25h; | 96.8 mg |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With ethyl acetate at 40℃; for 4h; | 99.64% |
-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With dihydro-(+)-lipoic acid; magnesium methanolate In isopropyl alcohol at 15 - 20℃; for 0.35h; Product distribution / selectivity; | 98.7% |
| With magnesium hydroxide In ethanol | 85% |
| With magnesium methanolate In methanol; isopropyl alcohol Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: bethanechol chloride With sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; Stage #2: (R)-1,2-dithiolane-3-pentanoic acid In methanol at 20℃; for 16h; | 97.6% |
-
-
1161826-45-4
3β-hydroxyoleanane-12-en-28-oic acid(6-bromohexyl) ester

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; | 96% |
-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol | 95% |
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
-
919507-94-1
R-(+)-LA dimethylethanolamine salt

| Conditions | Yield |
|---|---|
| In ethanol | 95% |
-
-
67-56-1
methanol

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
-
99427-00-6
(R)-5-[1,2-dithiolan-3-yl]pentanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid at 78.3℃; for 1h; Dean-Stark; | 95% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 5h; | |
| With thionyl chloride at 0 - 20℃; for 1h; | |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Cooling with ice; |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 25℃; for 19h; | 95% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N,N-dimethyl-formamide In dichloromethane at 20℃; | 94% |
| With dicyclohexyl-carbodiimide In 1,4-dioxane at 0 - 20℃; |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
-
874111-37-2
N-[(R)-1,2-dithiolane-3-pentanoyl]taurine

| Conditions | Yield |
|---|---|
| Stage #1: (R)-1,2-dithiolane-3-pentanoic acid With di(succinimido) carbonate; N-ethyl-N,N-diisopropylamine In acetone at 20℃; for 2h; Darkness; Stage #2: Taurine With water; N-ethyl-N,N-diisopropylamine In acetone | 95% |

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; 1,1'-carbonyldiimidazole In 1,2-dichloro-ethane at 20℃; for 24h; Cooling with ice; Green chemistry; | 95% |
| With 2,6-dimethylpyridine; 1,1'-carbonyldiimidazole In chloroform at 20℃; for 24h; Solvent; Reagent/catalyst; Temperature; | 85% |
-
-
851912-61-3
(2S,4R)-1-(6-aminohexanoyl)-4-hydroxy-2-(4,4′-dimethoxytrityloxymethyl)pyrrolidine

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (R)-1,2-dithiolane-3-pentanoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 0.166667h; Stage #2: (2S,4R)-1-(6-aminohexanoyl)-4-hydroxy-2-(4,4′-dimethoxytrityloxymethyl)pyrrolidine In N,N-dimethyl-formamide at 25℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 4h; Product distribution / selectivity; Reflux; | 94.5% |
| In acetone at 0 - 15℃; for 3h; | 92.3% |
| In acetone at 0 - 15℃; for 3h; | 92.3% |
| In acetone at 0 - 15℃; for 3h; | 92.3% |
-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

-
-
1115175-97-7
2,5-dioxopyrrolidin-1-yl 5-(1,2-dithiolan-3-yl)pentanoate

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 94% |
| With dicyclohexyl-carbodiimide In tetrahydrofuran; dichloromethane at 4 - 20℃; for 5h; | 75.8% |
| With dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 16h; | 66% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Inert atmosphere; |
-
-
1383539-92-1
bromohexyl-3β-hydroxy-urs-12-en-28-oate

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; Solvent; Reagent/catalyst; | 94% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; | 94% |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| In ethanol at 0 - 60℃; Product distribution / selectivity; | 93.1% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene at 35℃; for 18h; Cooling with ice; Green chemistry; | 93% |
| With dmap; dicyclohexyl-carbodiimide In chloroform at 35℃; for 18h; Solvent; Reagent/catalyst; Temperature; | 89% |

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 30℃; for 10h; | 92.1% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 35℃; for 18h; Cooling with ice; | 92% |
-
-
657-27-2, 10098-89-2
L-Lysine hydrochloride

-
-
1200-22-2
(R)-1,2-dithiolane-3-pentanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (R)-1,2-dithiolane-3-pentanoic acid With sodium ethanolate In ethanol at 20℃; Stage #2: L-Lysine hydrochloride In acetone at 55 - 60℃; for 2h; Concentration; Temperature; | 92% |
R-(+)-alpha-Lipoic acid Chemical Properties
IUPAC Name: 5-[(3R)-Dithiolan-3-yl]pentanoic acid
Following is the structure of R-(+)-alpha-Lipoic acid (CAS NO.1200-22-2):
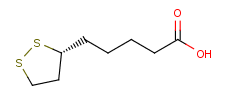
Empirical Formula: C8H14O2S2
Molecular Weight: 206.3256
Index of Refraction: 1.562
Molar Refractivity: 54.94 cm3
Molar Volume: 169.3 cm3
Density: 1.218 g/cm3
Flash Point: 173 °C
Melting point 46-49 °C
Surface Tension: 52.7 dyne/cm
Appearance: Yellow Crystalline Solid
Enthalpy of Vaporization: 66.83 kJ/mol
Boiling Point: 362.5 °C at 760 mmHg
Vapour Pressure of R-(+)-alpha-Lipoic acid (CAS NO.1200-22-2): 3.07E-06 mmHg at 25 °C
Product Categoriesof R-(+)-alpha-Lipoic acid (CAS NO.1200-22-2): FINE Chemical & INTERMEDIATES; Chiral Reagents; Heterocycles; Sulfur & Selenium Compounds; Chiral Building Blocks; Heterocyclic Building Blocks; Others
Canonical SMILES: C1CSSC1CCCCC(=O)O
Isomeric SMILES: C1CSS[C@@H]1CCCCC(=O)O
InChI: InChI=1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1
InChIKey: AGBQKNBQESQNJD-SSDOTTSWSA-N
R-(+)-alpha-Lipoic acid Uses
R-(+)-alpha-Lipoic acid (CAS NO.1200-22-2) can be used as a fat-metabolism stimulator .
R-(+)-alpha-Lipoic acid Specification
R-(+)-alpha-Lipoic acid , its cas register number 1200-22-2. It also can be called R-(+)-alpha-Lipoic acid ; and (R)-(+)-1,2-Dithiolane-3-pentanoic acid .
Related Products
- R-(+)-alpha-Lipoic acid
- 120022-63-1
- 12002-43-6
- 12002-48-1
- 1200-27-7
- 12002-82-3
- 12002-86-7
- 12003-37-1
- 12003-38-2
- 120034-05-1
- 12003-77-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View