-
Name
Stavudine
- EINECS 641-374-0
- CAS No. 3056-17-5
- Article Data76
- CAS DataBase
- Density 1.374 g/cm3
- Solubility Water solubility: 5-10 g/100 mL at 21 °C
- Melting Point 159-160 °C
- Formula C10H12N2O4
- Boiling Point
- Molecular Weight 224.216
- Flash Point
- Transport Information
- Appearance white powder
- Safety 26-S36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Thymidine,2',3'-didehydro-3'-deoxy-;Thymine,1-(2,3-dideoxy-b-D-glycero-pent-2-enofuranosyl)- (7CI,8CI);2'-Thymidinene, 3'-deoxy- (8CI);2',3'-Didehydro-3'-deoxythymidine;3'-Deoxy-2',3'-didehydrothymidine;Avostav;BMY 27857;D 4T (nucleoside);NSC163661;Stag;Stavir;Virostav;Zerit;d4T;
- PSA 84.32000
- LogP -0.70910
Synthetic route
-
-
125440-17-7
1-<5-O-(tert-butyldiphenylsilyl)-2,3-dideoxy-β-D-glycero-pento-2-enofuranosyl>thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 1.5h; Ambient temperature; | 100% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 25℃; | 90% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran Ambient temperature; | 78% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran Yield given; |
-
-
131933-47-6
1-<2',3'-dideoxy-3'(R)-phenylseleno-β-D-glycero-pentofuranosyl>thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In tetrahydrofuran 1) 0 deg C, 0.25 h, 2) warm to RT, 1 h; | 99% |
-
-
126209-27-6
1-(5-0-Pivaloyl-2,3-dideoxy-β-D-glycero-pent-2-enofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 23℃; for 4h; | 95% |
| With buffer In ethanol at 40℃; Rate constant; hydrolysis, also enzymatic hydrolysis; various pH-values; |
-
-
15981-92-7
2,3'-anhydrothymidine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With 1-(2-methoxyethyl)-3-methylimidazolium trifluoroacetate; sodium hydride In mineral oil at 100℃; for 0.0833333h; | 93% |
| With sodium hydride In N,N-dimethyl acetamide at 100℃; for 0.5h; | 81% |
-
-
122567-97-9
5'-O-benzoyl-2',3'-didehydro-3'-deoxythymidine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol Ambient temperature; | 92% |
| With N-butylamine at 70℃; for 6h; | 90% |
| With N-butylamine | 90% |
-
-
149666-71-7
1-{5-[bis(4-methoxyphenyl)phenyl-methoxymethyl]-2,5-dihydrofuran-2-yl}-5-methyl-1H-pyrimidine-2,4-dione

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| In methanol; tetrachloromethane at 25 - 40℃; for 3h; ultrasonic; | 92% |
| With methanol; Dowex 50W H(1+) form for 0.166667h; | 90% |

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 0 - 70℃; for 1.33333h; Purification / work up; | 91.4% |
| In water; acetone at 0℃; for 2.16667h; Purification / work up; | 76% |
| In water at 0 - 65℃; for 2.33333h; Purification / work up; | 73.7% |
-
-
938050-90-9
2',3'-didehydro-3'-deoxythymidine N,N-dimethyl propylene urea solvate

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| Stage #1: 2',3'-didehydro-3'-deoxythymidine N,N-dimethyl propylene urea solvate In water; isopropyl alcohol at 82℃; Stage #2: In isopropyl alcohol at 0 - 5℃; for 1.5h; Purification / work up; | 87.2% |
| In isopropyl alcohol at 0 - 82℃; for 1.5h; Product distribution / selectivity; | 87.2% |
| Stage #1: 2',3'-didehydro-3'-deoxythymidine N,N-dimethyl propylene urea solvate In water; acetone at 55℃; Stage #2: In acetone at 0 - 40℃; for 1h; Purification / work up; | 84% |
-
-
77421-68-2
1-(5-O-acetyl-2,3-dideoxy-β-D-glycero-pent-2-enofuranosyl)-thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 2h; Ambient temperature; | 87% |
| With sodium hydroxide In acetonitrile for 0.5h; Yield given; | |
| With buffer In ethanol at 40℃; Rate constant; hydrolysis, also enzymatic hydrolysis; various pH-values; |
-
-
131933-44-3
1-(2,3-dideoxy-3-C-selenophenyl-β-D-erythro-pentofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In tetrahydrofuran 1) 0 deg C, 0.25 h, 2) warm to RT, 1 h; | 86% |

-
-
102-82-9
tributyl-amine

-
-
1463-10-1
5-Methyluridine

-
-
149-73-5
trimethyl orthoformate

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With sodium methylate; acetic anhydride; sodium carbonate; hydrous zirconium oxide In methanol | 86% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide In tert-butyl alcohol at 55 - 60℃; for 2h; | 85% |
| With sodium hydride In N,N-dimethyl acetamide at 100℃; for 0.75h; | 76% |
| With potassium tert-butylate In dimethyl sulfoxide at 21℃; for 17h; | 57% |
-
-
119794-55-7
1-(5-O-tert-butyldimethylsilyl-2,3-dideoxy-β-D-glycero-pent-2-enofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran | 80% |
-
-
117383-83-2
5'-O-t-butyl-dimethylsilyl-3'-O-methanesulfonyl-thymidine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide for 0.5h; Ambient temperature; | 80% |

| Conditions | Yield |
|---|---|
| With acetic acid at 50℃; for 1h; | 71% |
| With acetic acid In water at 80 - 90℃; for 1h; | 67% |
| With acetic acid |
- 5'-O-(4-Methoxybenzyl)-2',3'-didehydro-2',3'-dideoxy-N3-(4-methoxybenzyl)-5-methyluridine
-
161824-93-7
5'-O-(4-Methoxybenzyl)-2',3'-didehydro-2',3'-dideoxy-N3-(4-methoxybenzyl)-5-methyluridine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile for 3h; Ambient temperature; | 68% |
-
-
4336-39-4
(2R,3R,4R,5R)-2-(acetoxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl diacetate

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3R,4R,5R)-2-(acetoxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl diacetate With palladium diacetate; lithium bromide In N,N-dimethyl-formamide at 180℃; for 36h; Inert atmosphere; Stage #2: With sodium methylate In methanol at 20℃; for 3h; Concentration; Temperature; Reagent/catalyst; Solvent; | 68% |
-
-
152039-45-7
1-(5-O-tert-butyldiphenylsilyl-2-deoxy-3-O-p-toluenesulfonyl-β-D-erythro-pentofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 48h; Heating; | 60% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 24h; Heating; Yield given; |
- 1-(3,5-di-O-acetyl-2-bromo-2-deoxy-β-D-ribofuranosyl)thymine
-
110483-43-7
1-(3,5-di-O-acetyl-2-bromo-2-deoxy-β-D-ribofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With copper; zinc In methanol for 0.5h; | 53% |
| Multi-step reaction with 2 steps 1: tetraethylammonium p-toluenesulfonate / methanol / 1 h / Electrolysis; Pt plate anode, Hg pool cathode 2: NH4OH View Scheme | |
| Multi-step reaction with 2 steps 1: 1.) zinc powder, 2.) aq. EDTA sodium salt / 1.) CH3CN, RT, 2 h 2: aq. NaOH / acetonitrile / 0.5 h View Scheme | |
| Multi-step reaction with 2 steps 1: 53 percent / Zn/Cu / methanol / 0.5 h 2: 87 percent / sodium methoxide / methanol / 2 h / Ambient temperature View Scheme |
-
-
139212-94-5, 139212-95-6
1-(5-O-tert-butyldiphenylsilyl-2-deoxy-3-O-methylsulfonyl-α,β-D-erythro-pentofuranosyl)thymine

-
A
-
15981-92-7
2,3'-anhydrothymidine

-
B
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 24h; Ambient temperature; | A 52% B 17% |
-
-
144867-91-4
1-(2',3'-dideoxy-2'-iodo-5'-O-(trimethylacetyl)-β-D-ribofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol | 48% |
| With sodium methylate In methanol at 20℃; for 18h; Yield given; | |
| Multi-step reaction with 3 steps 1: DBU / CH2Cl2 / 1 h / 0 °C 2: 82 percent / potassium t-butoxide / tetrahydrofuran / 1 h / 0 °C 3: 95 percent / sodium methoxide / methanol / 4 h / 23 °C View Scheme |
-
-
155807-21-9
1-(3,5-anhydro-2-deoxy-β-D-threo-pentofuranosyl)-2-O-ethylthymine

-
A
-
5983-11-9
2-methyl-5-(thymin-1-yl)furan

-
B
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide for 1h; Ambient temperature; | A 32.5% B 3.5% |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
7481-90-5
3',4'-anhydrothymidine

-
A
-
124685-30-9
1-((2R,4R,5R)-4-Hydroxy-5-pentyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione

-
B
-
65-71-4
thymin

-
C
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -70℃; for 1h; | A 31% B 18% C 13% |

-
-
594-19-4
tert.-butyl lithium

-
-
7481-90-5
3',4'-anhydrothymidine

-
A
-
124685-31-0
1-[(2R,4R,5R)-5-(2,2-Dimethyl-propyl)-4-hydroxy-tetrahydro-furan-2-yl]-5-methyl-1H-pyrimidine-2,4-dione

-
B
-
118778-33-9
1-(2,5-dideoxy-β-D-threo-pentofuranosyl)thymine

-
C
-
65-71-4
thymin

-
D
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In tetrahydrofuran; pentane at -70℃; for 1h; Yields of byproduct given; | A 26% B n/a C 27% D n/a |
| With boron trifluoride diethyl etherate In tetrahydrofuran; pentane at -70℃; for 1h; Yield given. Yields of byproduct given; | A 26% B n/a C 27% D n/a |
| With boron trifluoride diethyl etherate In tetrahydrofuran; pentane at -70℃; for 1h; Yield given; | A 26% B n/a C 27% D n/a |


| Conditions | Yield |
|---|---|
| With ammonia; copper; acetic acid; zinc 1.) DMF, RT, 30 min; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
42214-24-4
1-(2-deoxy-3-O-methanesulfonyl-5-O-trityl-β-D-ribopentofuranosyl)thymine

-
A
-
3056-17-5
stavudin

-
B
-
25526-93-6
alovudine

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; acetic acid 1.) THF, RT, 32 h, 2.) 110 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; | |
| With diethylamino-sulfur trifluoride; acetic acid 1.) THF, benzene, 2 h, 2.) 100 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
38313-48-3
1-(2-deoxy-3,5-epoxy-β-D-threo-pentofuranosyl)thymine

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide | |
| With potassium hydroxide In isopropyl alcohol Rearrangement; |

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With buffer In ethanol at 40℃; Rate constant; hydrolysis, also enzymatic hydrolysis; various pH-values; |
-
-
152336-78-2
2',3'-didehydro-3'-deoxythymidine 5'-O-hemisuccinate

-
-
3056-17-5
stavudin

| Conditions | Yield |
|---|---|
| With buffer In ethanol at 40℃; Rate constant; hydrolysis, also enzymatic hydrolysis; various pH-values; |
STAVUDINE Chemical Properties
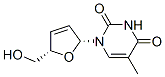
IUPAC Name:1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione
Molecular Formula:C10H12N2O4
Molecular Weight:224.213280:
Melting Point:159-160°C
refractive index:-46 ° (C=0.69, H2O)
storage temp.:20°C
Water Solubility:5-10 g/100 mL at 21°C
CAS DataBase Reference:3056-17-5(CAS DataBase Reference)
EPA Substance Registry System:3056-17-5(EPA Substance)
Synonyms of STAVUDINE(3056-17-5):
STAVUDINE;ZERIT;3'-deoxythymidin-2'-ene;3'-DEOXY-2',3'-DIDEHYDROTHYMIDINE;1-((2R,5S)-5-HYDROXYMETHYL-2,5-DIHYDRO-FURAN-2-YL)-5-METHYL-1H-PYRIMIDINE-2,4-DIONE;1-[2,3-DIDEOXY-BETA-D-GLYCERO-PENT-2-ENOFURANOSYL]THYMINE;2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE;2',3'-DIHYDRO-2',3'-DEOXYTHYMIDINE
Categories of STAVUDINE(3056-17-5):
Active Pharmaceutical Ingredients;chiral;Antivirals for Research and Experimental Use;Biochemistry;Chemical Reagents for Pharmacology Research;Nucleosides and their analogs;Nucleosides, Nucleotides & Related Reagents;Bases & Related Reagents;Nucleotides;Pharmaceuticals;API's
STAVUDINE History
STAVUDINE(3056-17-5) was the fourth antiretroviral drug on the market, its patent expired in the United States on 2008-06-25.
STAVUDINE(3056-17-5) was first synthetized in the sixties by Jerome Horwitz,subsequently reconsidered as an anti-HIV agent by the Rega Institute for Medical Research in Belgium.
STAVUDINE(3056-17-5) was approved by the U.S.FDA in Jun 24, 1994 for adults and in Sep 6, 1996 for pediatric use.
STAVUDINE(3056-17-5) was approved by the U.S.FDA for once-a-day dosing in 2001.
STAVUDINE Uses
STAVUDINE Toxicity Data With Reference
| 1. | dni-hmn-lym 20 µmol/L | MOPMA3 Molecular Pharmacology. 39 (1991),625. | ||
| 2. | cyt-orl-mus 600 mg/kg/3D-C | EMMUEG Environmental and Molecular Mutagenesis. 18 (1991),168. | ||
| 3. | orl-rat LD50:4 g/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),1944. | ||
| 4. | ivn-rat LD50:>1200 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),1944. | ||
| 5. | orl-mus LD50:1 g/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),1944. | ||
| 6. | ivn-mus LD50:1 g/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),1944. | ||
| 7. | orl-dog LD :>2 g/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),1944. | ||
| 8. | ivn-dog LD :>1680 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),1944. |
STAVUDINE Safety Profile
Safety Information of STAVUDINE(3056-17-5):
Hazard Codes:Xi 
Risk Statements:36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:26-36
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36:Wear suitable protective clothing
WGK Germany:2
RTECS:XP2075000
Hazardous Substances Data:3056-17-5(Hazardous Substances Data)
Moderately toxic by ingestion and intravenous routes. Mutation data reported. When heated to decomposition it emits toxic vapors of NOx.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View