-
Name
Sorbic acid
- EINECS 203-768-7
- CAS No. 110-44-1
- Article Data130
- CAS DataBase
- Density 1.024 g/cm3
- Solubility 1.6 g/L (20 ºC) in water
- Melting Point 132-135 ºC
- Formula C6H8O2
- Boiling Point 233 ºC at 760 mmHg
- Molecular Weight 112.128
- Flash Point 127 ºC
- Transport Information
- Appearance White crystalline powder
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms alpha-trans-gamma-trans-sorbic acid;(2E,4E)-hexa-2,4-dienoic acid;Hexadienoic acid, (E,E);2,4-Hexadienoic acid;trans,trans-sorbic acid;trans,trans-2,4-hexadienoic acid;hexa-2,4-dienoate;hexadienoic acid;hexa-2,4-dienoic acid;1,3-pentadiene-1-carboxylic acid;2,4-hexadienoic acid, (2E,4E)-;(2-butenylidene)acetic acid;crotylidene acetic acid;(E,E)-2,4-hexadienoic acid;2-Propenylacrylic acid;2,4-Hexadienoic acid,(2E,4E)-;2,4-Hexadienoic acid; Sorbic acid;2, 4—hexadienic acid;
- PSA 37.30000
- LogP 1.20330
Synthetic route
-
-
17102-64-6
Sorbyl alcohol

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With Acetobacter aceti MIM2000/28 In phosphate buffer; ethanol for 2h; pH=6.8; | 100% |

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| Stage #1: C12H22O2Si; carbon tetrabromide In methanol at 20℃; for 0.5h; Irradiation; Stage #2: In methanol at 20℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With C4H11FeMo6NO24(3-)*3C16H36N(1+); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 8h; Green chemistry; | 97% |
| With 4H3N*4H(1+)*CuMo6O18(OH)6(4-); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 12h; | 95% |
| With Acetobacter aceti MIM2000/28 In phosphate buffer; ethanol for 2h; pH=6.8; | 86% |
| With sodium hydroxide; triethanolamine; ethylene diamine tetraacetic acid tetrasodium salt Reagens 4:Silber;Leiten von Luft druch ein Gemisch; |

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| Stage #1: C15H28O2Si; carbon tetrabromide In methanol at 20℃; for 0.5h; Irradiation; Stage #2: In methanol at 20℃; for 2.5h; | 92% |

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| Stage #1: C22H26O2Si; carbon tetrabromide In methanol at 20℃; for 0.5h; Irradiation; Stage #2: In methanol at 20℃; for 5h; | 91% |
-
-
123-73-9
trans-Crotonaldehyde

-
-
65946-59-0
(trimethylsilyl)ketene bis(trimethylsilyl) acetal

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With zinc dibromide In tetrahydrofuran for 3h; Ambient temperature; | 85% |
-
-
77928-01-9
(E)-2-(Toluene-4-sulfonyloxy)-hex-4-enoic acid

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 25 - 30℃; for 1h; | 81% |

| Conditions | Yield |
|---|---|
| With pyridine Heating; | 80% |

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 20℃; | 77% |
-
-
27751-97-9
4-hydroxy-6-methyl-tetrahydro-2H-pyran-2-one

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| Amberlyst-70 In tetrahydrofuran; water at 169.84℃; under 15514.9 Torr; for 12h; Product distribution / selectivity; Inert atmosphere; | 64.1% |

-
A
-
110-44-1
sorbic Acid

-
B
-
51127-10-7
β-Amino-propionamidin

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 6h; Heating; | A 15% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With toluene; zinc bis(3-methylbutanoate) Erhitzen des dabei erhaltenen Reaktionsprodukts mit wenig Natriumhydroxid in Diaethylenglykol; | |
| With zinc disorbate Erhitzen des Reaktionsprodukts mit wss.Natronlauge; | |
| With toluene; zinc bis(3-methylbutanoate) Erhitzen des dabei erhaltenen Reaktionsprodukts ohne Zusatz auf 205grad; | |
| With toluene; zinc bis(3-methylbutanoate) Erhitzen des dabei erhaltenen Reaktionsprodukts mit wss.Natronlauge und anschliessend mit wss.Schwefelsaeure; |
-
-
56-23-5
tetrachloromethane

-
-
128-08-5
N-Bromosuccinimide

-
-
31124-99-9
6-methyl-2-oxo-tetrahydro-pyran-3-carboxylic acid ethyl ester

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| Erhitzen des mit N,N-Diaethyl-anilin auf 140grad und Erhitzen des Reaktionsprodukts mit wss. Natronlauge; |

-
-
31124-99-9
6-methyl-2-oxo-tetrahydro-pyran-3-carboxylic acid ethyl ester

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With tetrachloromethane; N-Bromosuccinimide Erhitzen des Reaktionsprodukts mit N,N-Diaethyl-anilin auf 140grad und Erhitzen des erhaltenen Sorbinsaeure-aethylesters mit wss.Natronlauge; |

| Conditions | Yield |
|---|---|
| at 140℃; Erhitzen des Reaktionsprodukts mit wss. Natronlauge; |
-
-
22229-95-4
(2Z)-2,5-hexadienoic acid

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With alkaline solution |
-
-
41143-12-8
hex-4-ynoic acid

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; ethylene glycol at 160℃; |

| Conditions | Yield |
|---|---|
| at 145℃; |
-
-
4423-18-1
(but-2-enylidene)malonic acid

-
A
-
110-44-1
sorbic Acid

-
B
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| bei der Kalischmelze; |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 50 - 60℃; und Destillation des Reaktionsprodukts unter vermindertem Druck; | |
| With boron trioxide; diethyl ether; oxalic acid at -15 - 15℃; und Erwaermen des Reaktionsprodukts mit 35prozentiger H2SO4 auf 70-80grad; | |
| With boron trioxide; diethyl ether; salicylic acid at -15 - 15℃; und Erwaermen des Reaktionsprodukts mit 35prozentiger H2SO4 auf 70-80grad; |
-
-
123-73-9
trans-Crotonaldehyde

-
-
141-82-2
malonic acid

-
A
-
110-44-1
sorbic Acid

-
B
-
110-44-1, 5309-56-8, 5309-57-9, 22500-92-1, 30361-30-9
(2Z,4E)-hexa-2,4-dienoic acid

| Conditions | Yield |
|---|---|
| With pyridine at 98℃; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
4423-18-1
(but-2-enylidene)malonic acid

-
A
-
110-44-1
sorbic Acid

-
B
-
110-44-1, 5309-56-8, 5309-57-9, 22500-92-1, 30361-30-9
(2Z,4E)-hexa-2,4-dienoic acid

| Conditions | Yield |
|---|---|
| With pyridine at 100℃; for 3h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide for 0.666667h; Heating; | 100% |
| With thionyl chloride; N,N-dimethyl-formamide at 80℃; for 0.666667h; Inert atmosphere; | 98% |
| With thionyl chloride In N,N-dimethyl-formamide at 80℃; for 0.666667h; Inert atmosphere; | 98% |
-
-
110-44-1
sorbic Acid

-
-
32775-95-4
(E)-3,5-hexadienoic acid

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide for 1h; Ambient temperature; | 100% |
| With lithium diisopropyl amide In tetrahydrofuran for 1h; Ambient temperature; | 100% |
| With n-butyllithium; diisopropylamine In tetrahydrofuran at -10 - 20℃; for 1h; | 98% |
-
-
292638-84-7
styrene

-
-
110-44-1
sorbic Acid

-
-
66-25-1
hexanal

-
-
142077-75-6, 142077-76-7, 142247-75-4, 142247-76-5, 142247-77-6
(1RS,2SR)-2-(1-hydroxyhexyl)-3,5-hexadienoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water; diisopropylamine; pentane | 100% |

-
-
110-44-1
sorbic Acid

-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
98453-85-1
(2E,4E)-2,5-dioxopyrrolidin-1-yl hexa-2,4-dienoate

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 2.5h; Inert atmosphere; | 100% |
-
-
110-44-1
sorbic Acid


| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at -10℃; for 2h; Reflux; Inert atmosphere; | 99% |
| With chloro-trimethyl-silane In dichloromethane for 16h; | 99% |
| Stage #1: sorbic Acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 0℃; for 1h; Inert atmosphere; Stage #2: methanol In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; | 95% |
-
-
110-44-1
sorbic Acid

-
-
23283-97-8
(+)-(1S,2R,5R)-isomenthol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 3h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| tetrachlorobis(tetrahydrofuran)hafnium(IV) In benzene for 38h; Heating; | 99% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 12.0833h; | 94% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 48h; | 90% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 48h; | 90% |
| With mineral acid Heating; | 40.2% |
-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| Stage #1: Wang resin-bound Fmoc-L-Tyr(t-Bu) With piperidine In N,N-dimethyl-formamide at 20℃; Stage #2: sorbic Acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide at 20℃; for 18h; Stage #3: With trifluoroacetic acid In dichloromethane at 20℃; for 18h; | 99% |
-
-
110-44-1
sorbic Acid

-
-
1352928-05-2
(6S,7S,12S,E)-6-hydroxy-12-isopropyl-7,9-dimethyloxacyclododec-9-en-2-one

-
-
1352928-06-3
(6S,7S,12S,E)-12-isopropyl-7,9-dimethyl-2-oxooxacyclododec-9-en-6-yl 4,15-dioxo-19-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)-8,11-dioxa-5,14-diazanonadecan-1-oate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 15h; Höfle-Steglich esterification; | 99% |
-
-
110-44-1
sorbic Acid


| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1.5h; | 99% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; Inert atmosphere; | 99% |
-
-
110-44-1
sorbic Acid

-
-
2640-94-0
methyl 10-hydroxydecanoate

-
-
1314897-83-0
methyl 10-((2E,4E)-hexa-2,4-dienoyloxy)decanoate

| Conditions | Yield |
|---|---|
| Stage #1: sorbic Acid With 2,4,6-trichlorobenzoyl chloride; triethylamine In dichloromethane at 20℃; Yamaguchi reaction; Cooling with ice; Stage #2: methyl 10-hydroxydecanoate With dmap In dichloromethane at 20℃; Yamaguchi reaction; | 98% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; | 98% |
-
-
110-44-1
sorbic Acid

-
-
17102-64-6
Sorbyl alcohol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 1h; Heating; | 97% |
| Stage #1: sorbic Acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 0.5h; Stage #2: With sodium tetrahydroborate; water In tetrahydrofuran at 0℃; for 0.5h; | 75% |
| With strain of the zygomycete fungus S. racemosum MUT 770 In dimethyl sulfoxide for 72h; Enzymatic reaction; | 74% |
-
-
110-44-1
sorbic Acid

-
-
416856-95-6
(2S,3R)-2-((2E,4E)-Hexa-2,4-dienoylamino)-3-hydroxy-butyric acid tert-butyl ester

-
-
416856-96-7
(2E,4E)-Hexa-2,4-dienoic acid (1R,2S)-2-tert-butoxycarbonyl-2-((2E,4E)-hexa-2,4-dienoylamino)-1-methyl-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; 4-(dimethylamino)pyridine hydrochloride; dacarbazine In dichloromethane at 20℃; for 6h; | 97% |
-
-
110-44-1
sorbic Acid

-
-
555-31-7
aluminum isopropoxide

-
-
57377-08-9
aluminium(III) sorbate bis(isopropoxide)

| Conditions | Yield |
|---|---|
| In benzene byproducts: isoprpopanol; to Al(OCH(CH3)2)3 in benzene was added acid (molar ratio 1:1), mixt. was heated under reflux; i-PrOH was fractionated, solvent was removed under reduced pressure; elem. anal.; | 97% |
-
-
110-44-1
sorbic Acid

-
-
115171-26-1
(E)-3-(4-Methoxy-7-methyl-bicyclo[4.2.0]octa-1(6),2,4-trien-7-yl)-but-2-en-1-ol

-
-
115171-48-7
(2E,4E)-Hexa-2,4-dienoic acid (E)-3-(4-methoxy-7-methyl-bicyclo[4.2.0]octa-1(6),2,4-trien-7-yl)-but-2-enyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide | 96% |
-
-
110-44-1
sorbic Acid

-
-
150-19-6
O-methylresorcine

-
-
170120-19-1
3-methoxyphenyl (2E,4E)-2,4-hexanedienoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 24h; | 96% |
-
-
110-44-1
sorbic Acid

-
-
416856-95-6
(2S,3R)-2-((2E,4E)-Hexa-2,4-dienoylamino)-3-hydroxy-butyric acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 5h; | 96% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: isoprpopanol; to Al(OCH(CH3)2)3 in benzene was added acid (molar ratio 1:2), mixt. was heated under reflux; i-PrOH was fractionated, solvent was removed under reduced pressure; elem. anal.; | 96% |
-
-
110-44-1
sorbic Acid


| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1.5h; | 96% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: sorbic Acid With thionyl chloride In methanol at 0℃; for 3h; Reflux; Inert atmosphere; Stage #2: Inert atmosphere; | 95% |
| Multi-step reaction with 2 steps 1: phosphorus pentachloride / man fraktioniert das Produkt im Vakuum View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: sorbic Acid With 4-(dimethylamino)pyridine N-oxide; phenylboronic acid In fluorobenzene at 20℃; for 0.0833333h; Molecular sieve; Reflux; Stage #2: phenethylamine In fluorobenzene for 16h; Molecular sieve; Reflux; | 95% |
-
-
110-44-1
sorbic Acid

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1.5h; | 95% |
Sorbic acid Chemical Properties
IUPAC Name: (2E,4E)-hexa-2,4-dienoic acid
Empirical Formula: C6H8O2
Molecular Weight: 112.1265
EINECS: 203-768-7
Structure of Sorbic acid (CAS NO.110-44-1):
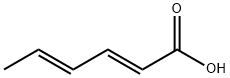
Index of Refraction: 1.487
Molar Refractivity: 31.52 cm3
Molar Volume: 109.4 cm3
Polarizability: 12.49×10-24cm3
Surface Tension: 35.4 dyne/cm
Density: 1.024 g/cm3
Flash Point: 139.9 °C
Enthalpy of Vaporization: 51.74 kJ/mol
Melting Point: 132-135 °C(lit.)
Boiling Point: 233 °C at 760 mmHg
Vapour Pressure: 0.02 mmHg at 25°C
Storage temp: 2-8°C
Solubility: ethanol: 0.1 g/mL, clear
Water Solubility: 1.6 g/L (20 ºC)
Stability: Stability Material saturated with this acid may ignite spontaneously. Incompatible with strong oxidizing agents. May be light sensitive.
Product Categories: FOOD ADDITIVES;Food and Feed Additive;Food & Feed ADDITIVES;Fatty & Aliphatic Acids, Esters, Alcohols & Derivatives;Organic acids
Canonical SMILES: CC=CC=CC(=O)O
Isomeric SMILES: C/C=C/C=C/C(=O)O
InChI: InChI=1S/C6H8O2/c1-2-3-4-5-6(7)8/h2-5H,1H3,(H,7,8)/b3-2+,5-4+
InChIKey: WSWCOQWTEOXDQX-MQQKCMAXSA-N
Sorbic acid History
SORBIC ACID was first isolated from the unripe berries of the Rowan (Sorbus aucuparia), hence its name.
Sorbic acid Uses
Sorbic acid (CAS NO.110-44-1) and its mineral salts, such as sodium sorbate, potassium sorbate and calcium sorbate, are antimicrobial agents often used as preservatives in food and drinks to prevent the growth of mold, yeast and fungi.
Sorbic acid (CAS NO.110-44-1) is also used as a bio descaling agent.
Sorbic acid Toxicity Data With Reference
| 1. | skn-man 150 mg/1H SEV | JPPMAB Journal of Pharmacy and Pharmacology. 10 (1958),719. | ||
| 2. | skn-rbt 1 mg SEV | UCDS** Union Carbide Data Sheet. (Industrial Medicine and Toxicology Dept., Union Carbide Corp., 270 Park Ave., New York, NY 10017) 7/14 ,1965. | ||
| 3. | cyt-ham:lng 1050 mg/L | FCTOD7 Food and Chemical Toxicology. 22 (1984),501. | ||
| 4. | sce-ham:lng 1050 mg/L | FCTOD7 Food and Chemical Toxicology. 22 (1984),501. | ||
| 5. | scu-rat TDLo:1040 mg/kg/65W-I:ETA | BJCAAI British Journal of Cancer. 20 (1966),134. | ||
| 6. | orl-rat LD50:7360 mg/kg | JIHTAB Journal of Industrial Hygiene and Toxicology. 30 (1948),63. | ||
| 7. | orl-mus LD50:3200 mg/kg | 38MKAJ Pattys Industrial Hygiene and Toxicology. 2C (1982),4953. | ||
| 8. | ipr-mus LD50:2820 mg/kg | JPPMAB Journal of Pharmacy and Pharmacology. 21 (1969),85. | ||
| 9. | scu-mus LD50:2820 mg/kg | JPPMAB Journal of Pharmacy and Pharmacology. 21 (1969),85. |
Sorbic acid Consensus Reports
Reported in EPA TSCA Inventory.
Sorbic acid Safety Profile
Moderately toxic by intraperitoneal and subcutaneous routes. Mildly toxic by ingestion. Experimental reproductive effects. A severe human and experimental skin irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:  Xi
Xi
The Risk Statements information of Sorbic acid :
36/37/38: Irritating to eyes, respiratory system and skin
36/38: Irritating to eyes and skin
The Safety Statements information of Sorbic acid :
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
24/25: Avoid contact with skin and eyes
WGK Germany:1
Sorbic acid Specification
Sorbic acid , its cas register number is 110-44-1. It also can be called (E,E)-2,4-Hexadienoic acid ; 1,3-Pentadiene-1-carboxylic acid, (E,E)- ; 2,4-Hexadienoic acid, (E,E)- ; 2-Propenylacrylic acid ; 2E,4E-Hexadienoic acid ; Acetic acid, (2-butenylidene)- ; Acetic acid, crotylidene- ; EPA Pesticide Chemical Code 075901 ; Hexadienoic acid ; Kyselina 1,3-pentadien-1-karboxylova ; Kyselina sorbova ; Panosorb ; Sorbistat ; alpha-trans-gamma-trans-Sorbic acid ; trans,trans-Sorbic acid ; trans-trans-2,4-Hexadienoic acid . Sorbic acid (CAS NO.110-44-1) is a white powder or crystals.
Related Products
- Sorbic acid
- SORBIC ACID VINYL ESTER
- 110-45-2
- 110453-78-6
- 1104606-44-1
- 110-46-3
- 110464-98-7
- 11046-99-4
- 110471-15-3
- 110-47-4
- 110475-31-5
- 1104786-91-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View