-
Name
Tetrahydrofuran
- EINECS 203-726-8
- CAS No. 109-99-9
- Article Data381
- CAS DataBase
- Density 0.904 g/cm3
- Solubility miscible with water
- Melting Point 33-36 ºC
- Formula C4H8O
- Boiling Point 68.3 ºC at 760 mmHg
- Molecular Weight 72.1069
- Flash Point -21 ºC
- Transport Information UN 2924 3/PG 2
- Appearance Colorless liquid
- Safety 26-36-33-29-16
- Risk Codes 36/37/38-19-11
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, F
F
- Synonyms NCI-C60560;Polytetrahydrofuran;Oxacyclopentane;Tetramethylene oxide;Hydrofuran;Tetrahydrofuraan;Tetraidrofurano;Butane .alpha.,.delta.-oxide;Oxolane;Tetrahydrfuran;Furan, tetrahydro-;Tetrahydrofuranne;THF;Cyclotetramethylene oxide;Furan,tetrahydro-;Furanidine;Diethylene oxide;Butane, 1,4-epoxy-;Tetra hydro furan;
- PSA 9.23000
- LogP 0.79680
Synthetic route

| Conditions | Yield |
|---|---|
| With 3% Pd/C; hydrogen In isopropyl alcohol at 219.84℃; under 25858.1 Torr; for 5h; Inert atmosphere; | 100% |
| With hydrogen; acetic acid In water at 39.84℃; for 2h; Inert atmosphere; | 98% |
| With ruthenium; hydrogen; 1-butyl-3-methylimidazolium Tetrafluoroborate at 25℃; under 22502.3 Torr; for 36h; Autoclave; chemoselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With phosphoric acid; 5%-palladium/activated carbon; hydrogen at 100℃; under 3750.38 - 13501.4 Torr; for 7h; Reagent/catalyst; Pressure; Temperature; Autoclave; | 100% |

| Conditions | Yield |
|---|---|
| Trichlorbutylstannan at 80 - 84℃; for 9h; | 99% |
| Trichlorbutylstannan at 80 - 84℃; for 19h; Mechanism; different molar ratios, different times; | 99% |
| zirconium(IV) sulfate at 200℃; under 760.051 Torr; Product distribution / selectivity; Gas phase; | 99.5% |

| Conditions | Yield |
|---|---|
| Stage #1: n-butane With oxygen at 403℃; under 2175.22 Torr; Stage #2: With hydrogen In Phthalic acid dibutyl ester Product distribution / selectivity; | 99.5% |
-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
110-15-6
succinic acid

-
E
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5 percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.37% B 0.28% C 0.37% D 98.89% E 0.08% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
67-56-1
methanol

-
D
-
110-63-4
Butane-1,4-diol

-
E
-
617-48-1
malic acid

-
F
-
110-15-6
succinic acid

-
G
-
64-19-7
acetic acid

-
H
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.45% B 0.06% C 0% D 0.21% E 0.36% F 98.73% G 0.04% H 0.08% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
591-81-1
4-hydroxybutanoic acid

-
E
-
110-15-6
succinic acid

-
F
-
64-19-7
acetic acid

-
G
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.77% B 0.38% C 0.24% D 0.05% E 98.28% F 0.02% G 0.26% |


| Conditions | Yield |
|---|---|
| With perchloric acid; sodium perchlorate; water at 25℃; | A n/a B n/a C 98% |

-
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
A
-
109-99-9
tetrahydrofuran

-
B
-
13436-45-8
2-methoxytetrahydrofuran

-
C
-
96-48-0
4-butanolide

-
D
-
71-23-8
propan-1-ol

-
E
-
64001-06-5
2-(4'-hydroxybutoxy)-tetrahydrofuran

-
F
-
110-63-4
Butane-1,4-diol

-
G
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; copper catalyst, T 4489, Sud-Chemie AG, Munich at 150 - 280℃; under 187519 Torr; Neat liquid(s) and gas(es)/vapour(s); | A 1% B n/a C 0.4% D n/a E n/a F 98% G 0.5% |


| Conditions | Yield |
|---|---|
| With water In toluene byproducts: hydrogen, dihydronaphthalene; reaction time: 20 h; centrifuged, decanted, pptn. (Yb(OH)3) washed with toluene, soln. contains THF and C10H10 (82%)(detn. by GLC); | A 112 % B 98% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sodium formate; Aliquat 336 at 105℃; for 0.25h; | 95% |

| Conditions | Yield |
|---|---|
| A n/a B 94.2% | |
| A n/a B 94.2% |


| Conditions | Yield |
|---|---|
| decompn. at 80°C (0.5 h); | A 93% B n/a |
| decompn. at 80°C (0.5 h); | A 93% B n/a |
-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
591-81-1
4-hydroxybutanoic acid

-
E
-
617-48-1
malic acid

-
F
-
110-15-6
succinic acid

-
G
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd/2.0percent Re on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 1.27% B 4.78% C 1.55% D 1.24% E 0.48% F 90.6% G 0.08% |


-
A
-
109-99-9
tetrahydrofuran

-
B
-
523981-77-3
cyclopentadienylterbiumdibromide

| Conditions | Yield |
|---|---|
| In neat (no solvent) warmed at 40°C under high vac. for 24 h; elem. anal.; | A n/a B 90% |
-
-
110-15-6
succinic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 139.84℃; under 60006 Torr; for 96h; Catalytic behavior; Reagent/catalyst; Time; Temperature; Autoclave; Overall yield = 100 %; | A 0.2% B 3.1% C 89% D 7.6% |
| With hydrogen; 1.0percent Pd/ 3.0percent Re on Rutile TiO2 at 164 - 185℃; for 21 - 237h; Product distribution / selectivity; | A 2.95% B 0% C 81.5% D 3.35% |
| With hydrogen; 0percent Pd/5.0percent Re on Rutile TiO2 at 170 - 185℃; for 90 - 825h; Product distribution / selectivity; | A 3.38% B 0% C 64.14% D 2.86% |

-
-
110-15-6
succinic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
107-92-6
butyric acid

-
E
-
106-97-8
n-butane

-
F
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 139.84℃; under 60006 Torr; for 24h; Catalytic behavior; Reagent/catalyst; Time; Autoclave; Overall yield = > 99 %; | A 0.2% B 3.1% C 89% D n/a E n/a F 7.6% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
617-48-1
malic acid

-
E
-
110-15-6
succinic acid

-
F
-
64-19-7
acetic acid

-
G
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; for 96 - 238h; Product distribution / selectivity; | A 0.6% B 0.04% C 0.62% D 0.19% E 88.49% F 0.12% G 0.11% |


| Conditions | Yield |
|---|---|
| With hydrogen at 350℃; for 0.6h; Reagent/catalyst; Temperature; Flow reactor; | A n/a B 85.9% |
| With Mg and Yb-containing organic foam into the binaryoxides at 350℃; Reagent/catalyst; Temperature; Inert atmosphere; | A n/a B 71.1% |
| With Er2O3 nanoparticles CM-1000 at 350℃; for 5h; | |
| at 350℃; Reagent/catalyst; Flow reactor; Inert atmosphere; |
-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
591-81-1
4-hydroxybutanoic acid

-
E
-
617-48-1
malic acid

-
F
-
110-15-6
succinic acid

-
G
-
100-21-0
terephthalic acid

-
H
-
64-19-7
acetic acid

-
I
-
802294-64-0
propionic acid

-
J
-
110-17-8
(2E)-but-2-enedioic acid

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd/0.2percent Re on Rutile TiO2 at 110℃; for 170 - 1009h; Product distribution / selectivity; | A 0.86% B 4.34% C 0.28% D 1.24% E 0% F 85.51% G 0% H 0.04% I 0% J 0% |

-
-
23055-10-9
1,4-dimethyl but-2-enedioate

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In water at 130 - 182℃; under 52505.3 Torr; | A 6.34% B 5.33% C 83.96% |

-
-
88-14-2
2-furanoic acid

-
-
64-19-7
acetic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 6% B 83% |

-
-
96-48-0
4-butanolide

-
A
-
109-99-9
tetrahydrofuran

-
B
-
71-23-8
propan-1-ol

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
107-92-6
butyric acid

-
E
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 5% platinum on alumina at 250℃; under 152000 Torr; Product distribution; var. catalysts; | A 82.3% B 1% C 4.8% D 1.2% E 1% |

-
-
79372-14-8
decamethylsamarocene(II) bis(tetrahydrofurane)

-
-
75-24-1
trimethylaluminum

-
A
-
109-99-9
tetrahydrofuran

-
B
-
115756-72-4
(C5Me5)2Sm{(μ-Me)AlMe2(μ-Me)}2Sm(C5Me5)2

-
C
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| In toluene byproducts: methane; all manipulations conducted under nitrogen excluding air and water; after 24 h standing of the reaction mixt. the formed metallic-like ppt. was removed by filtration and washed with hot toluene, filtrates combined, solvent removed by rotary evapn.;; recrystn. (hot toluene), elem. anal.;; | A n/a B 80% C n/a |

-
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
A
-
109-99-9
tetrahydrofuran

-
B
-
13436-45-8
2-methoxytetrahydrofuran

-
C
-
96-48-0
4-butanolide

-
D
-
71-23-8
propan-1-ol

-
F
-
64001-06-5
2-(4'-hydroxybutoxy)-tetrahydrofuran

-
H
-
110-63-4
Butane-1,4-diol

-
I
-
25714-71-0
4-hydroxybutyraldehyde

-
J
-
925-57-5
methyl 4-hydroxybutanoate

-
K
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen at 190℃; under 46504.7 Torr; Gas phase; | A 5.3% B n/a C 10.4% D n/a E n/a F n/a G n/a H 79.1% I n/a J n/a K n/a |

-
-
110-00-9
furan

-
-
67-56-1
methanol

-
A
-
109-99-9
tetrahydrofuran

-
B
-
13179-96-9
1,4-dimethoxybutane

-
C
-
107-31-3
Methyl formate

-
D
-
29006-01-7
Methyl 4-methoxybutyrate

-
E
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen at 170℃; under 52505.3 Torr; for 2h; Autoclave; | A 77.2% B n/a C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen at 130 - 182℃; under 52505.3 Torr; | A 13.09% B 77% |

| Conditions | Yield |
|---|---|
| decompn. at 140°C, <760 Torr; | A 75% B n/a |
-
-
110-63-4
Butane-1,4-diol

-
-
616-38-6
carbonic acid dimethyl ester

-
A
-
109-99-9
tetrahydrofuran

-
B
-
140947-75-7
butane-1,4-diyl dimethyl dicarbonate

| Conditions | Yield |
|---|---|
| With sodium methylate Reflux; Inert atmosphere; | A 12 %Chromat. B 75% |

-
-
109-99-9
tetrahydrofuran

-
-
33036-62-3
4-Bromo-1-butanol

| Conditions | Yield |
|---|---|
| With dimethylboron bromide; triethylamine In dichloromethane at 0℃; for 2h; | 100% |
| With sulfuric acid; hydrogen bromide | 90% |
| With tetrabutylammomium bromide; hydrogen bromide In water for 0.0833333h; Microwave irradiation; | 81% |

| Conditions | Yield |
|---|---|
| nickel at 100℃; for 1h; | 100% |
| With [(POCOP)Ir(H)(acetone)]+[B(C6F5)4]- In dichloromethane-d2 at 22℃; for 3h; | 100 %Spectr. |

| Conditions | Yield |
|---|---|
| With zinc(II) iodide In dichloromethane for 72h; Product distribution; other cyclic ethers; other Lewis acids; | 100% |
| With zinc(II) iodide In dichloromethane for 72h; | 100% |

| Conditions | Yield |
|---|---|
| With calcium for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With bis(iodozinc)methane at 25℃; for 2h; Substitution; | 100% |
| With bis(iodozinc)methane; lead(II) chloride at 25℃; for 2h; | 99% |
| With sodium iodide In acetonitrile at 0 - 23℃; for 24h; | 99.3% |
-
-
109-99-9
tetrahydrofuran

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
92511-12-1
1-{[dimethyl(1,1-dimethylethyl)silyl]oxy}-4-iodobutane

| Conditions | Yield |
|---|---|
| With sodium iodide In acetonitrile at 55℃; for 16h; Inert atmosphere; | 100% |
| With sodium iodide In tetrahydrofuran; acetonitrile at 55℃; for 18h; Inert atmosphere; | 100% |
| With sodium iodide In acetonitrile at 20℃; Inert atmosphere; Darkness; | 93% |
-
-
109-99-9
tetrahydrofuran

-
-
68602-57-3
trifluoroacetyl triflate

-
-
109244-09-9
Trifluoro-acetic acid 4-trifluoromethanesulfonyloxy-butyl ester

| Conditions | Yield |
|---|---|
| at 0℃; | 100% |

| Conditions | Yield |
|---|---|
| With bis(iodozinc)methane at 25℃; Substitution; | 100% |

| Conditions | Yield |
|---|---|
| With bis(iodozinc)methane at 25℃; Substitution; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
625-35-4, 3488-22-0, 10487-71-5
trans-chrotonyl chloride

| Conditions | Yield |
|---|---|
| With bis(iodozinc)methane at 25℃; Substitution; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
102-92-1
Cinnamoyl chloride

-
-
143903-00-8
(E)-3-Phenyl-acrylic acid 4-iodo-butyl ester

| Conditions | Yield |
|---|---|
| With bis(iodozinc)methane at 25℃; Substitution; | 100% |

| Conditions | Yield |
|---|---|
| With bis(iodozinc)methane at 25℃; Substitution; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
217965-97-4
2,2,4,4,9,9,11,11,13,13,15,15,17,17,21,21-hexadecamethyl-2,4,9,11,13,15,17,21-octasilahexacyclo[10.5.3.15,8.06,18.07,20.014,19]henicosa-1(18),5,7,12(20),14(19)-pentaene

| Conditions | Yield |
|---|---|
| With lithium at 20℃; for 1h; Reduction; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
87653-00-7
trichloro(4,5-dihydrofuran-3-yl)phosphonium hexachlorophosphate

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In benzene at 10 - 20℃; for 2h; Substitution; | 100% |
| With phosphorus pentachloride In benzene at 10 - 20℃; for 2h; Inert atmosphere; | 100% |
-
-
109-99-9
tetrahydrofuran

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: dichloro(mesityl)phosphane; C14H20BrMgP*C4H10O at -78℃; Stage #2: tetrahydrofuran With lithium for 1h; sonication; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: tetrahydrofuran; N,N,N,N,-tetramethylethylenediamine; diphenylphosphane With n-butyllithium In tetrahydrofuran; hexane Stage #2: With tellurium In tetrahydrofuran; hexane at -78 - 20℃; | 100% |


| Conditions | Yield |
|---|---|
| With sodium hydride at 60℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydride at 60℃; for 12h; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
13368-25-7
1-(bromomethyl)-4-vinylbenzene

- 4-vinylbenzyl-polytetrahydrofuran, with hydroxyl end group, polymerization degree 7.1 by 1H NMR, polydispersity 1.1; monomer(s): 4-vinylbenzyl bromide; tetrahydrofuran
-
4-vinylbenzyl-polytetrahydrofuran, with hydroxyl end group, polymerization degree 7.1 by 1H NMR, polydispersity 1.1; monomer(s): 4-vinylbenzyl bromide; tetrahydrofuran

| Conditions | Yield |
|---|---|
| Stage #1: tetrahydrofuran; 1-(bromomethyl)-4-vinylbenzene With silver(I) hexafluorophosphate at -10℃; for 0.133333h; Stage #2: With sodium hydroxide In water | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| With potassium borohydride for 528h; Heating; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
213380-73-5
bis(α-trifluoromethyl-β,β-difluorovinyl) terephthalate

| Conditions | Yield |
|---|---|
| at 0℃; Irradiation; | 100% |
| at 0℃; Kinetics; Irradiation; |
-
-
109-99-9
tetrahydrofuran

-
-
395099-09-9
(2-amino-5-(benzyloxy)phenyl)(phenyl)methanone

| Conditions | Yield |
|---|---|
| In methanol; water | 100% |
| In methanol; water | 100% |
| In methanol; water | 100% |

-
-
109-99-9
tetrahydrofuran

-
-
40125-53-9
2-bromo-5-oxo-2,5-dihydrofuran

-
-
1030603-64-5
5-(4-bromobutoxy)furan-2(5H)-one

| Conditions | Yield |
|---|---|
| With zinc dibromide In dichloromethane for 4h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran W compd. dissolve in THF, stirred for 15 min, under anaerobic anhydrousconditions; elem. anal.; | 100% |
-
-
109-99-9
tetrahydrofuran

-
-
299410-39-2
Zr(C6H5NCH2CH2CH2NC6H5)2

-
-
10026-11-6
zirconium(IV) chloride

-
-
299410-33-6
Zr(PhN(CH2)3NPh)Cl2(THF)2

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: LiCl; N2 atm.; THF and diethyl ether were added by vac. transfer at -78°C to the mixt. of complex and ZrCl4, the mixt. was warmed to 0°Cin an ice bath, stirred overnight, allowed to warm to room temp.; volatiles were removed under vac. at 23°C; elem. anal.; | 100% |

-
-
109-99-9
tetrahydrofuran

-
-
354112-52-0
dicarbonyl[tris(pyrazol-1-yl)methanesulfonato]rhodium(I)

-
-
603-35-0
triphenylphosphine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran all manipulations under N2; P compd. added to soln. of complex in THF; after 1 h soln. concd., soln. overlaid with pentane, elem. anal.; | 100% |

-
-
109-99-9
tetrahydrofuran

-
-
168979-95-1
(Sm(OC15H23)2(O(C2H5)2))2(OC13H8)2

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); quantitative conversion; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (high vac. line); condensing gallium complex in an ampoule with THF, warming to room temp. over a period of 30 min; fractionation, collection in a trap at -20°C; | 100% |
Tetrahydrofuran Consensus Reports
NTP Carcinogenesis Bioassay (feed); Clear Evidence: mouse, Some Evidence: rat NTPTR* National Toxicology Program Technical Report Series. (Research Triangle Park, NC 27709) No. NTP-TR-475 ,1998. . Reported in EPA TSCA Inventory.
Tetrahydrofuran Standards and Recommendations
OSHA PEL: TWA 200 ppm; STEL 250 ppm
ACGIH TLV: TWA 200 ppm; STEL 250 ppm; BEI: 8 mg/L tetrahydrofuran in urine at end of shift
DFG MAK: 50 ppm (150 mg/m3)
DOT Classification: 3; Label: Flammable Liquid
Tetrahydrofuran Analytical Methods
Tetrahydrofuran (CAS NO.109-99-9), its occupational chemical analysis use NIOSH: Tetrahydrofuran, 1609.
Tetrahydrofuran Specification
Tetrahydrofuran (THF) is an organic compound with the formula (CH2)4O. It is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. The compound is heterocyclic. As one of the most polar ethers with a wide liquid range, it is a useful solvent. With the CAS NO.109-99-9, it is also called 1,4-Epoxybutane ; AI3-07570 ; Agrisynth THF ; Butane, 1,4-epoxy- ; Butane, alpha,delta-oxide ; Butylene oxide ; CCRIS 6276 ; Cyclotetramethylene oxide ; Diethylene oxide.
Physical properties about Tetrahydrofuran are: (1)ACD/LogP: 0.473; (2)ACD/LogD (pH 5.5): 0.47; (3)ACD/LogD (pH 7.4): 0.47; (4)ACD/BCF (pH 5.5): 1.35; (5)ACD/BCF (pH 7.4): 1.35; (6)ACD/KOC (pH 5.5): 43.07; (7)ACD/KOC (pH 7.4): 43.07; (8)#H bond acceptors: 1; (9)Index of Refraction: 1.417; (10)Molar Refractivity: 20.052 cm3; (11)Molar Volume: 79.76 cm3; (12)Polarizability: 7.949 10-24cm3; (13)Surface Tension: 28.8099994659424 dyne/cm; (14)Density: 0.904 g/cm3; (15)Flash Point: -17.222 °C; (16)Enthalpy of Vaporization: 29.81 kJ/mol; (17)Boiling Point: 68.278 °C at 760 mmHg; (18)Vapour Pressure: 152.442993164063 mmHg at 25°C
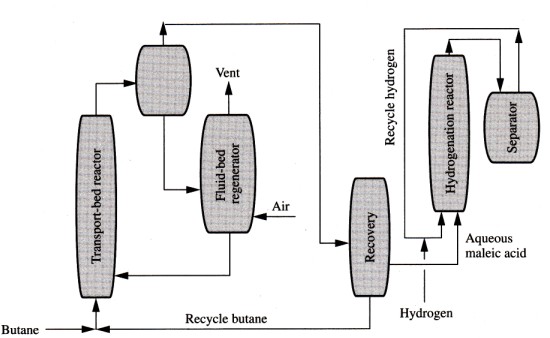
Preparation of Tetrahydrofuran: Tetrahydrofuran (CAS NO.109-99-9) can be manufactured from butane by using circulating solids technology in which butane is oxidized to maleic acid and thence to tetrahydrofuran
Uses of Tetrahydrofuran: The main application of Tetrahydrofuran (CAS NO.109-99-9) is as an industrial solvent for PVC and in varnishes.
Tetrahydrofuran(CAS NO.109-99-9) can be polymerized by strong acids to give a linear polymer called poly(tetramethylene ether) glycol (PTMEG), also known as PTMO, polytetramethylene oxide. The primary use of this polymer is to make elastomeric polyurethane fibers like Spandex. THF can be used in hydroboration reactions to synthesize primary alcohols, and as a solvent for organometallic compounds such as organolithium and Grignard reagents. It is often used in polymer science. It can be used to liquefy old PVC cement, and is often used industrially to degrease metal parts.
When you are using this chemical, please be cautious about it as the following:
1. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
2. Wear suitable protective clothing;
3. Take precautionary measures against static discharges;
4. Do not empty into drains;
5. Keep away from sources of ignition - No smoking;
You can still convert the following datas into molecular structure:
(1)InChI=1S/C4H8O/c1-2-4-5-3-1/h1-4H2;
(2)InChIKey=WYURNTSHIVDZCO-UHFFFAOYSA-N;
(3)SmilesC1CCOC1
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 2300mg/kg (2300mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 34(9), Pg. 114, 1969. | |
| guinea pig | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | LIVER: FATTY LIVER DEGERATION | American Industrial Hygiene Association Journal. Vol. 35, Pg. 21, 1974. |
| human | TCLo | inhalation | 25000ppm (25000ppm) | BEHAVIORAL: GENERAL ANESTHETIC | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 580, 1969. |
| mouse | LCLo | inhalation | 24000mg/m3/2H (24000mg/m3) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: MUSCLE WEAKNESS | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 5, Pg. 21, 1963. |
| mouse | LD50 | intraperitoneal | 1900mg/kg (1900mg/kg) | Sangyo Igaku. Japanese Journal of Industrial Health. Vol. 24, Pg. 373, 1982. | |
| rabbit | LC | inhalation | > 1200ppm/4H (1200ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION | Sumitomo Sangyo Eisei. Sumitomo Industrial Health. Vol. 18, Pg. 89, 1982. |
| rat | LC50 | inhalation | 21000ppm/3H (21000ppm) | BEHAVIORAL: SLEEP GASTROINTESTINAL: NAUSEA OR VOMITING LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Sumitomo Sangyo Eisei. Sumitomo Industrial Health. Vol. 20, Pg. 141, 1984. |
| rat | LD50 | intraperitoneal | 2900mg/kg (2900mg/kg) | Sangyo Igaku. Japanese Journal of Industrial Health. Vol. 24, Pg. 373, 1982. | |
| rat | LD50 | oral | 1650mg/kg (1650mg/kg) | GAF Material Safety Data Sheet. |
Related Products
- Tetrahydrofuran
- Tetrahydrofuran-2,3,4,5-tetracarboxylic dianhydride
- Tetrahydrofuran-2,5-dicarboxylic acid
- Tetrahydrofuran-2-carboxylic acid (3-methylaminopropyl)amide
- Tetrahydrofuran-3-carboxylic acid
- Tetrahydrofuran-3-ylamine hydrochloride
- 11000-17-2
- 11000-26-3
- 110003-22-0
- 110-00-9
- 110-01-0
- 1100-10-3
- 110011-77-3
- 110013-18-8
- 110013-19-9
- 110-02-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View