-
Name
Triamcinolone
- EINECS 204-718-7
- CAS No. 124-94-7
- Density 1.42 g/cm3
- Solubility 79.99mg/L(25 oC)
- Melting Point 262-263 °C(lit.)
- Formula C21H27FO6
- Boiling Point 587.5 °C at 760 mmHg
- Molecular Weight 394.44
- Flash Point 309.1 °C
- Transport Information
- Appearance
- Safety 22-36
- Risk Codes 40
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 11b,16a,17a,21-Tetrahydroxy-9a-fluoro-1,4-pregnadiene-3,20-dione;9-Fluoro-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione;9a-Fluoro-16a-hydroxyprednisolone;Adcortyl;CL 19823;Cinolone;Fluoxyprednisolone;Kenacort;Ledercort;Mycolog;NSC13397;Omicilon;Polcortolon;Rodinolone;Triamcinlon;Triamcinolone;Tricortale;Volon;
- PSA 115.06000
- LogP 0.62050
Synthetic route
-
-
3859-65-2
(11β,16α)-21-acetyloxy-9-fluoro-11,16,17-trihydroxypegna-1,4-diene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; dichloromethane at -7 - -3℃; Inert atmosphere; | 85.29% |

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Stage #1: C28H38O9 With hydrogen fluoride In chloroform; water at -40℃; Stage #2: With toluene-4-sulfonic acid at 40℃; | 81% |

| Conditions | Yield |
|---|---|
| mit Hilfe von Streptomyces roseochromogenus; |
-
-
4001-36-9
16β,21-diacetoxy-9-fluoro-11β,17-dihydroxy-pregna-1,4-diene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
98422-57-2, 114417-77-5
16β,21-diacetoxy-9,11β-epoxy-17-hydroxy-9β-pregn-4-ene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: CH2Cl2; THF; HF 2: SeO2 3: methanol.KOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: SeO2 2: methanol.KOH View Scheme |
-
-
98422-57-2, 114417-77-5
16α,21-diacetoxy-9,11β-epoxy-17-hydroxy-9β-pregn-4-ene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: CHCl3; HF 2: mit Hilfe von Nocardia corallina 3: methanol.KOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: mit Hilfe von Corynebacterium simplex oder Nocardia corallina 2: pyridine 3: SOCl2; pyridine 4: aqueous HClO4; dioxane 5: potassium acetate; ethanol 6: CHCl3; HF 7: methanol.KOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: mit Hilfe von Nocardia corallina 2: methanol.KOH View Scheme |
-
-
13951-70-7
desonide

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: pyridine 2: SOCl2; pyridine 3: aqueous HClO4; dioxane 4: potassium acetate; ethanol 5: CHCl3; HF 6: methanol.KOH View Scheme |
-
-
23460-76-6
21-acetoxy-pregna-4,9(11),16-triene-3,20-dinone

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: KMnO4 2: pyridine 3: aqueous HClO4; dioxane 4: potassium acetate; ethanol / Behandeln einer Loesung des Reaktionsprodukts in Pyridin mit Acetanhydrid 5: CHCl3; HF 6: mit Hilfe von Nocardia corallina 7: methanol.KOH View Scheme |
-
-
74220-43-2
21-Acetoxy-16α,17α-dihydroxy-4,9(11)-pregnadien-3,20-dion

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: pyridine 2: aqueous HClO4; dioxane 3: potassium acetate; ethanol / Behandeln einer Loesung des Reaktionsprodukts in Pyridin mit Acetanhydrid 4: CHCl3; HF 5: mit Hilfe von Nocardia corallina 6: methanol.KOH View Scheme |
-
-
95943-95-6
16α,21-diacetoxy-17-hydroxy-pregna-1,4,9(11)-triene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: aqueous HClO4; dioxane 2: potassium acetate; ethanol 3: CHCl3; HF 4: methanol.KOH View Scheme |
-
-
98422-55-0
16α,21-diacetoxyprednisolone

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: SOCl2; pyridine 2: aqueous HClO4; dioxane 3: potassium acetate; ethanol 4: CHCl3; HF 5: methanol.KOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: SeO2 2: SOCl2; pyridine 3: aqueous HClO4; dioxane 4: potassium acetate; ethanol 5: CHCl3; HF 6: methanol.KOH View Scheme |
-
-
91160-85-9
16α,21-diacetoxy-9-bromo-11β,17-dihydroxy-pregn-4-ene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium acetate; ethanol / Behandeln einer Loesung des Reaktionsprodukts in Pyridin mit Acetanhydrid 2: CHCl3; HF 3: mit Hilfe von Nocardia corallina 4: methanol.KOH View Scheme |
-
-
91160-86-0
16α,21-diacetoxy-9-bromo-11β,17-dihydroxy-pregna-1,4-diene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium acetate; ethanol 2: CHCl3; HF 3: methanol.KOH View Scheme |
-
-
98632-54-3
16α,21-diacetoxy-17-hydroxy-pregna-4,9(11)-diene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: aqueous HClO4; dioxane 2: potassium acetate; ethanol / Behandeln einer Loesung des Reaktionsprodukts in Pyridin mit Acetanhydrid 3: CHCl3; HF 4: mit Hilfe von Nocardia corallina 5: methanol.KOH View Scheme |
-
-
96670-24-5
16α,21-diacetoxy-9,11β-epoxy-17-hydroxy-9β-pregna-1,4-diene-3,20-dione

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CHCl3; HF 2: methanol.KOH View Scheme |
-
-
894767-41-0
triamcinolone phosphate

-
-
124-94-7
triamcinolone

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: formic acid; potassium permanganate / acetone; water / 1 h / -7 - -3 °C 2: hydrogen fluoride / -30 - -25 °C 3: sodium hydroxide / methanol; dichloromethane / -7 - -3 °C / Inert atmosphere View Scheme |

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen fluoride / -30 - -25 °C 2: sodium hydroxide / methanol; dichloromethane / -7 - -3 °C / Inert atmosphere View Scheme |
-
-
37413-91-5
2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; perchloric acid / acetone / -10 - -5 °C 1.2: 33 - 37 °C 2.1: formic acid; potassium permanganate / acetone; water / 1 h / -7 - -3 °C 3.1: hydrogen fluoride / -30 - -25 °C 4.1: sodium hydroxide / methanol; dichloromethane / -7 - -3 °C / Inert atmosphere View Scheme |
-
-
117048-56-3
17-acetyl-10,13-dimethyl-6,7,8,10,12,13,14,15-octahydrocyclopenta[a]phenathren-3

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydroxide / methanol / Darkness; Cooling with ice 1.2: 20 °C 2.1: potassium permanganate; formic acid / acetone / -5 - 5 °C 3.1: triethylamine; dmap / chloroform 4.1: 5,5-dibromohydantoin; perchloric acid / acetone / -5 - 5 °C 5.1: hydrogen fluoride / chloroform; water / -40 °C 5.2: 40 °C View Scheme |

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium permanganate; formic acid / acetone / -5 - 5 °C 2.1: triethylamine; dmap / chloroform 3.1: 5,5-dibromohydantoin; perchloric acid / acetone / -5 - 5 °C 4.1: hydrogen fluoride / chloroform; water / -40 °C 4.2: 40 °C View Scheme |

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine; dmap / chloroform 2.1: 5,5-dibromohydantoin; perchloric acid / acetone / -5 - 5 °C 3.1: hydrogen fluoride / chloroform; water / -40 °C 3.2: 40 °C View Scheme |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at -10 - 0℃; | 93% |
| With hydrogenchloride for 1h; Heating; | 52% |
| With methanesulfonic acid at 50℃; for 2h; Temperature; |
-
-
64-18-6
formic acid

-
-
124-94-7
triamcinolone

-
-
2878-64-0
9α-Fluor-16α,21-bis(formyloxy)-11β,17α-dihydroxy-1,4-pregnadien-3,20-dion

| Conditions | Yield |
|---|---|
| for 16h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With Wilkinson's catalyst; hydrogen In ethanol; toluene at 20℃; under 760 Torr; for 48h; Catalytic hydrogenation; | 88% |
| (tris(triphenylphosphine)rhodium) chloride In ethanol; dichloromethane; toluene |
-
-
124-94-7
triamcinolone

-
-
130063-07-9
9α-fluoro-11β,16α,17α-trihydroxy-17β-hydroxymethyl-D-homoandrosta-1,4-diene-3,17a-dione

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate In N,N-dimethyl-formamide at 55 - 60℃; for 4h; | 69% |
-
-
124-94-7
triamcinolone

-
-
159210-07-8
9α-fluoro-11β,16α,17α-trihydroxy-3-oxoandrosta-1,4-diene-17β-carboxylic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In methanol; N,N-dimethyl-formamide for 23h; | 45% |

| Conditions | Yield |
|---|---|
| at 7 - 8℃; | 40% |
-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; magnesium sulfate In acetonitrile at -10℃; | 39% |
-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| With perchloric acid In tetrahydrofuran at 20 - 25℃; for 2h; | 25.8% |
-
-
124-94-7
triamcinolone

-
A
-
130063-07-9
9α-fluoro-11β,16α,17α-trihydroxy-17β-hydroxymethyl-D-homoandrosta-1,4-diene-3,17a-dione

-
B
-
156352-67-9
(11β,16β,17α)-9-fluoro-11,16,17-trihydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione

| Conditions | Yield |
|---|---|
| With edetate disodium; sodium carbonate In methanol for 22h; Mechanism; Ambient temperature; also in the presence of transition metal salts, also triamcinolone 21 acetate; | A n/a B 22% |
-
-
124-94-7
triamcinolone

-
-
156352-67-9
(11β,16β,17α)-9-fluoro-11,16,17-trihydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione

| Conditions | Yield |
|---|---|
| With edetate disodium; sodium carbonate In methanol for 22h; | 22% |
-
-
124-94-7
triamcinolone

-
-
3869-38-3
16α,17-((Ξ)-ethylLiDenedioxy)-9-fluoro-11β,21-dihydroxy-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid; paracetaldehyde anschliessend Acetylierung und Hydrolyse; |

| Conditions | Yield |
|---|---|
| With perchloric acid; acetone | |
| With hydrogenchloride; acetone |
-
-
124-94-7
triamcinolone

-
-
3793-07-5
16α,17-((Ξ)-sec-butylidenedioxy)-9-fluoro-11β,21-dihydroxy-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid; butanone |
-
-
124-94-7
triamcinolone

-
-
3924-70-7
16α,17-(1-ethyl-propylidenedioxy)-9-fluoro-11β,21-dihydroxy-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid; pentan-3-one |
-
-
124-94-7
triamcinolone

-
-
3092-82-8
9-fluoro-11β,21-dihydroxy-16α,17-((Ξ)-1-phenyl-ethylLiDenedioxy)-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid; acetophenone |
-
-
124-94-7
triamcinolone

-
-
108-94-1
cyclohexanone

-
-
2794-88-9
16α,17-cyclohexyLiDenedioxy-9-fluoro-11β,21-dihydroxy-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol |
-
-
1445-45-0
Trimethyl orthoacetate

-
-
124-94-7
triamcinolone

-
-
3925-42-6
16α-Acetoxy-9α-fluor-11β,17α,21-trihydroxy-pregna-1,4-dien-3,20-dion

| Conditions | Yield |
|---|---|
| (i) aq. HClO4, MeOH, dioxane, (ii) aq. oxalic acid, MeOH; Multistep reaction; |
-
-
13820-09-2
trimethyl orthovalerate

-
-
124-94-7
triamcinolone

| Conditions | Yield |
|---|---|
| (i) aq. HClO4, MeOH, dioxane, (ii) aq. oxalic acid, MeOH; Multistep reaction; |
-
-
124-94-7
triamcinolone

-
-
51354-79-1
(trimethoxymethyl)cyclopentane

| Conditions | Yield |
|---|---|
| (i) aq. HClO4, MeOH, dioxane, (ii) aq. oxalic acid, MeOH; Multistep reaction; |
-
-
124-94-7
triamcinolone

-
-
108-10-1
4-methyl-2-pentanone

-
A
-
2794-91-4
16,17-(1,3-dimethyl-butylidenedioxy)-9-fluoro-11,21-dihydroxy-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid stereoisomer(ic) of mp: 258 degree; |

-
-
124-94-7
triamcinolone

-
-
108-10-1
4-methyl-2-pentanone

-
A
-
2794-91-4
16,17-(1,3-dimethyl-butylidenedioxy)-9-fluoro-11,21-dihydroxy-pregna-1,4-diene-3,20-dione

| Conditions | Yield |
|---|---|
| With perchloric acid stereoisomer(ic) of mp: 188 degree; |

-
-
124-94-7
triamcinolone

-
-
409094-09-3
Pentanedioic acid mono-[2-((4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-fluoro-5-hydroxy-4a,6a,8,8-tetramethyl-2-oxo-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodecahydro-7,9-dioxa-pentaleno[2,1-a]phenanthren-6b-yl)-2-oxo-ethyl] ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 52 percent / HCl / 1 h / Heating 2: 83 percent / pyridine / 18 h / 20 °C View Scheme |
Triamcinolone Chemical Properties
Molecular Formula: C21H27FO6
Molar mass: 394.44 g/mol
EINECS: 204-718-7
Density: 1.42 g/cm3
Flash Point: 309.1 °C
Index of Refraction: 1.617
Boiling Point: 587.5 °C at 760 mmHg
Vapour Pressure: 3E-16 mmHg at 25°C
Melting point: 260-263 ºC (dec.)
Synonyms of Triamcinolone (124-94-7): (8S,9R,10S,11S,13S,14S,16R,17S)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one ; (1R,2S,10S,11S,13R,14S,15S,17S)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one
Structure of Triamcinolone (124-94-7):
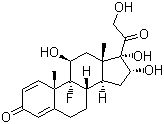
XLogP3-AA: 1.2
H-Bond Donor: 4
H-Bond Acceptor: 7
IUPAC Name: (8S,9R,10S,11S,13S,14S,16R,17S)-9-Fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
Canonical SMILES: CC12CC(C3(C(C1CC(C2(C(=O)CO)O)O)CCC4=CC(=O)C=CC43C)F)O
Isomeric SMILES: C[C@]12C[C@@H]([C@]3([C@H]([C@@H]1C[C@H]([C@@]2(C(=O)CO)O)O)CCC4=CC(=O)C=C[C@@]43C)F)O
InChI: InChI=1S/C21H27FO6/c1-18-6-5-12(24)7-11(18)3-4-13-14-8-15(25)21(28,
17(27)10-23)19(14,2)9-16(26)20(13,18)22/h5-7,13-16,23,25-26,28H,3-4,8-10H2,1-2H3/t13-,14-,15+,16-,18-,19-,20-,21-/m0/s1
InChIKey: GFNANZIMVAIWHM-OBYCQNJPSA-N
Triamcinolone Uses
Triamcinolone (124-94-7) is used in the post-operative period of certain cosmetic surgery procedures, notably rhinoplasty. Injected into the subcutaneous area, it may help to alleviate stubborn swelling, scar tissue, and inflamed sebaceous cysts.It is occasionally used to treat lichen planus and severe cases of hayfever, and may also be used to treat diabetic retinopathy.It is highly effective in the most severe cases of eczema, but may require a waiting period before subsequent treatments.Inaddition, it can be used in case of severe arthritis, Lumbago sciatica.
Triamcinolone Toxicity Data With Reference
| 1. | oms-hmn-orl 428 µg/kg | ARDEAC Archives of Dermatology. 103 (1971),39. | ||
| 2. | scu-rat LD50:99 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 8 (1966),250. |
Triamcinolone Safety Profile
Poison by subcutaneous route. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of F−. An anti-inflammatory and antiallergic agent.
Hazard Codes: Xn![]()
Risk Statements:
40: Limited evidence of a carcinogenic effect
Safety Statements:
22: Do not breathe dust
36: Wear suitable protective clothing
Triamcinolone Specification
Triamcinolone (124-94-7) (trade names Kenalog, Aristocort, Nasacort, Tri-Nasal, Triderm, Azmacort, Trilone, Volon A, Tristoject, Fougera, Tricortone,Triesence) is a synthetic corticosteroid given orally, by inhalation, injection, or as a topical ointment or cream.
Its side effects include nosebleeds, a sore throat, increased headache, coughing, and runny nose. White patches in the throat or nose indicates a serious side effect. Symptoms of an allergic reaction include itch, rash, severe dizziness, swelling, trouble breathing.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View