-
Name
Voriconazole
- EINECS 629-701-5
- CAS No. 137234-62-9
- Article Data35
- CAS DataBase
- Density 1.42 g/cm3
- Solubility
- Melting Point 127-130 °C
- Formula C16H14F3N5O
- Boiling Point 508.6 °C at 760 mmHg
- Molecular Weight 349.315
- Flash Point 261.4 °C
- Transport Information
- Appearance cyrstalline solid
- Safety 26-36
- Risk Codes 22-36/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 4-Pyrimidineethanol, alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1H-1,2,4-triazol-1-ylmethyl)-,(alphaR,betaS)-;DRG-0301;UK-109,496;UNII-JFU09I87TR;Vfend;VRC;
- PSA 76.72000
- LogP 2.17690
Synthetic route
-
-
188416-34-4, 137234-71-0
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (1R)-10-camphorsulfonate

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane for 0.5h; | 92% |
| With sodium hydrogencarbonate In water; ethyl acetate | 90.47% |
| With sodium hydroxide In water at 45 - 55℃; for 2.5h; Product distribution / selectivity; | 87.22% |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water pH=11 - 12; | 92% |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane for 0.5h; | 92% |
| With sodium hydroxide In dichloromethane; water at 15 - 25℃; pH=10 - 11; |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane for 0.5h; | 90.8% |
| With sodium hydroxide In dichloromethane; water pH=12.3; Product distribution / selectivity; Alkaline aqueous solution; Industry scale; | 72% |
| With sodium hydroxide In dichloromethane; water pH=11; Product distribution / selectivity; Alkaline aqueous solution; |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen In ethanol under 760.051 Torr; | 86.3% |
-
-
188416-38-8
(2R,3S)-3-(6-chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1 yl)butan-2-ol

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; potassium formate In ethanol; water at 30℃; for 1h; Temperature; Reagent/catalyst; Inert atmosphere; | 84.9% |
| With palladium 10% on activated carbon; hydrogen; sodium acetate In water; toluene at -21 - 40℃; under 3750.38 Torr; for 22h; |
-
-
848469-32-9
(2R,3S)-2-(2,4-diflurophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1yl)butan-2-ol R-(-)-10-camphor sulphonate salt

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane; water at 20℃; for 0.333333h; pH=~ 10; Product distribution / selectivity; | 76% |
| With sodium hydroxide In dichloromethane; water pH=11 - 12; Product distribution / selectivity; |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With water In ethanol at 32.5℃; Concentration; Solvent; Temperature; | 76% |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With [(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]methanesulfonic acid In methanol; acetone for 2h; Reflux; | 43% |
| With (R)-10-camphorsulfonic acid In methanol; acetone at 0 - 5℃; Resolution of racemate; | 40.1% |
| Multi-step reaction with 2 steps 1: acetone; methanol / 15 h / -20 - 25 °C 2: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme | |
| Multi-step reaction with 2 steps 1: methanol; acetone / Reflux 2: sodium hydroxide / water; dichloromethane / pH 11 View Scheme |
-
-
1602485-93-7
5-fluoro-4-vinylpyrimidine

-
-
86404-63-9
1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazolyl)ethanone

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With CuF(PPh3)3 methanol solvate; (R)-(−)-1-[(R)-2-(2′-diphenylphosphinophenyl)ferrocenyl]ethylbis(di-3,5-trifluoromethylphenyl)phosphine at 0℃; for 21.5h; Reagent/catalyst; Inert atmosphere; | 40% |
-
-
188416-35-5
3-(6-chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Stage #1: 3-(6-chloro-5-fluoropyrimidin-4-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol With 5%-palladium/activated carbon; hydrogen; sodium acetate In methanol at 25℃; under 760.051 Torr; Stage #2: With [(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]methanesulfonic acid In methanol; acetone for 2h; Reflux; | 35% |
| Multi-step reaction with 3 steps 1.1: sodium acetate / ethyl acetate; water / 0.5 h / 25 - 30 °C 1.2: 25 - 30 °C / 3677.86 Torr 1.3: 0.5 h / 20 - 25 °C 2.1: acetone; methanol / 15 h / -20 - 25 °C 3.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydrogen; sodium acetate / palladium 10% on activated carbon / ethyl acetate; water / 25 - 30 °C / 3677.86 Torr 1.2: 0.5 h / 20 - 25 °C 1.3: 12.5 h / 25 - 30 °C 2.1: sodium hydroxide / dichloromethane; water / 0.5 h / pH 9 - 12 3.1: acetone; methanol / 15 h / -20 - 25 °C 4.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydrogen; sodium acetate; sodium carbonate / palladium 10% on activated carbon / ethyl acetate; water / 25 - 30 °C / 3677.86 Torr 1.2: 0.5 h / 20 - 25 °C 1.3: 10 - 25 °C 2.1: potassium carbonate / dichloromethane; water / 0.5 h / pH 8 - 10 3.1: acetone; methanol / 15 h / -20 - 25 °C 4.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme |
-
-
288-88-0
1,2,4-Triazole

-
-
1474024-68-4
4-((S)-1-((R)-2-(2,4-difluorophenyl)oxirane-2-yl)ethyl)-5-fluoropyrimidine

-
A
-
1028563-65-6
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(4H-1,2,4-triazol-4-yl)butan-2-ol

-
C
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; for 18h; Inert atmosphere; | A 27% B n/a C n/a |

-
-
1003706-87-3
4-bromo-5-fluoro-pyrimidine

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -70 - 30℃; for 25h; Temperature; Inert atmosphere; | 26.3% |

-
-
347418-42-2
2,4-dichloro-5-fluoropyrimidine

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -70 - 30℃; for 25h; Temperature; Inert atmosphere; | 20.7% |

-
B
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; palladium on activated charcoal In ethanol at 20℃; |
-
-
759-67-1
2-fluoro-3-oxopentanoic acid ethyl ester

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1) MeONa, MeOH 2: POCl3 / Heating 3: LDA / tetrahydrofuran 4: H2, AcONa / Pd/C / ethanol / 20 °C View Scheme |
-
-
137234-87-8
6-Ethyl-5-fluoropyrimidin-4(3H)-one

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: POCl3 / Heating 2: LDA / tetrahydrofuran 3: H2, AcONa / Pd/C / ethanol / 20 °C View Scheme |
-
-
137234-74-3
4-chloro-5-fluoro-6-ethylpyrimidine

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: LDA / tetrahydrofuran 2: H2, AcONa / Pd/C / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: diisopropylamine; n-butyllithium / n-heptane; hexane; tetrahydrofuran / 0.25 h / -80 - -70 °C / Inert atmosphere 1.2: 2 h / -80 - -55 °C 1.3: -70 - -10 °C 2.1: sodium acetate / ethyl acetate; water / 0.5 h / 25 - 30 °C 2.2: 25 - 30 °C / 3677.86 Torr 2.3: 0.5 h / 20 - 25 °C 3.1: acetone; methanol / 15 h / -20 - 25 °C 4.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme | |
| Multi-step reaction with 5 steps 1.1: diisopropylamine; n-butyllithium / n-heptane; hexane; tetrahydrofuran / 0.25 h / -80 - -70 °C / Inert atmosphere 1.2: 2 h / -80 - -55 °C 1.3: -70 - -10 °C 2.1: hydrogen; sodium acetate / palladium 10% on activated carbon / ethyl acetate; water / 25 - 30 °C / 3677.86 Torr 2.2: 0.5 h / 20 - 25 °C 2.3: 12.5 h / 25 - 30 °C 3.1: sodium hydroxide / dichloromethane; water / 0.5 h / pH 9 - 12 4.1: acetone; methanol / 15 h / -20 - 25 °C 5.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme |
-
-
321589-01-9
2-(2,4difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)-butan-2-ol-R(-)-10-camphor sulphonate

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water at 25 - 35℃; pH=11 - 12; Alkaline conditions; |
-
-
188416-28-6
1-(4-chloro-5-fluoropyrimidin-6-yl)bromoethane

-
-
86404-63-9
1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazolyl)ethanone

-
B
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-chloro-5-fluoropyrimidin-6-yl)bromoethane; 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazolyl)ethanone With zinc(II) chloride; zinc In tetrahydrofuran at -5 - 5℃; for 1h; Stage #2: With ammonium formate; zinc In methanol at 20℃; for 16h; Stage #3: Product distribution / selectivity; Heating / reflux; |


-
B
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With ammonium formate; zinc In methanol Product distribution / selectivity; |

-
B
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate at 20 - 25℃; |
-
-
182230-43-9
2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol With (R)-10-camphorsulfonic acid In methanol; acetone at 25 - 60℃; for 2h; Stage #2: With sodium hydroxide In dichloromethane; water; isopropyl alcohol at 20 - 30℃; pH=12 - 13; Product distribution / selectivity; | |
| With (1S)-10-camphorsulfonic acid In methanol; acetone at 20 - 30℃; for 3.5h; Reflux; | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / water / 3 h / 45 °C 2: camphor-10-sulfonic acid / methanol; acetone View Scheme |
-
-
1289559-75-6
C6H5FN2O

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium tetrahydroborate / ethanol / 5 h 2.1: triethylamine / dmap / dichloromethane / 20 - 30 °C 3.1: zinc; zinc(II) chloride / tetrahydrofuran / 0 - 30 °C 4.1: (R)-10-camphorsulfonic acid / acetone; methanol / 2 h / 25 - 60 °C 4.2: 20 - 30 °C / pH 12 - 13 View Scheme |
-
-
1289559-65-4
1-(2,6-dichloro-5-fluoropyrimidin-4-yl)ethanone

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogen; sodium acetate / palladium on activated carbon / ethanol / 25 °C 2.1: sodium tetrahydroborate / ethanol / 5 h 3.1: triethylamine / dmap / dichloromethane / 20 - 30 °C 4.1: zinc; zinc(II) chloride / tetrahydrofuran / 0 - 30 °C 5.1: (R)-10-camphorsulfonic acid / acetone; methanol / 2 h / 25 - 60 °C 5.2: 20 - 30 °C / pH 12 - 13 View Scheme |
-
-
1289559-66-5
1-(5-fluoropyrimidin-4-yl)ethanol

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dmap / dichloromethane / 20 - 30 °C 2.1: zinc; zinc(II) chloride / tetrahydrofuran / 0 - 30 °C 3.1: (R)-10-camphorsulfonic acid / acetone; methanol / 2 h / 25 - 60 °C 3.2: 20 - 30 °C / pH 12 - 13 View Scheme |
-
-
51336-94-8
2-chloro-1-(2,4-dichlorophenyl)ethanone

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate / tetrahydrofuran / 7 h / 20 °C 2: lead; zinc; iodine / tetrahydrofuran / -15 - 20 °C / Inert atmosphere 3: ammonium formate / palladium on carbon / toluene / 3 h / 80 °C 4: acetone; methanol / 1 h / Reflux 5: sodium hydrogencarbonate / dichloromethane / 0.5 h View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / tetrahydrofuran / 7 h / 20 °C 2: lead; zinc; iodine / tetrahydrofuran / 2 h / -15 - -10 °C / Inert atmosphere 3: ammonium formate / 10% palladium on charcoal / toluene / 3 h / 80 °C 4: acetone; methanol / 1 h / Reflux 5: sodium hydrogencarbonate / dichloromethane / 0.5 h View Scheme |
-
-
137234-87-8
6-ethyl-5-fluoro-4-hydroxypyrimidine

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: triethylamine / dichloromethane / 5 h / 20 °C 2: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / dichloromethane / 12 h / 45 - 50 °C 3: lead; zinc; iodine / tetrahydrofuran / -15 - 20 °C / Inert atmosphere 4: ammonium formate / palladium on carbon / toluene / 3 h / 80 °C 5: acetone; methanol / 1 h / Reflux 6: sodium hydrogencarbonate / dichloromethane / 0.5 h View Scheme | |
| Multi-step reaction with 5 steps 1.1: triethylamine / dichloromethane / 5 - 10 °C 1.2: 8 h / 10 - 55 °C / Reflux 1.3: 0.5 h / 15 - 20 °C 2.1: diisopropylamine; n-butyllithium / n-heptane; hexane; tetrahydrofuran / 0.25 h / -80 - -70 °C / Inert atmosphere 2.2: 2 h / -80 - -55 °C 2.3: -70 - -10 °C 3.1: sodium acetate / ethyl acetate; water / 0.5 h / 25 - 30 °C 3.2: 25 - 30 °C / 3677.86 Torr 3.3: 0.5 h / 20 - 25 °C 4.1: acetone; methanol / 15 h / -20 - 25 °C 5.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme | |
| Multi-step reaction with 6 steps 1.1: triethylamine / dichloromethane / 5 - 10 °C 1.2: 8 h / 10 - 55 °C / Reflux 1.3: 0.5 h / 15 - 20 °C 2.1: diisopropylamine; n-butyllithium / n-heptane; hexane; tetrahydrofuran / 0.25 h / -80 - -70 °C / Inert atmosphere 2.2: 2 h / -80 - -55 °C 2.3: -70 - -10 °C 3.1: hydrogen; sodium acetate / palladium 10% on activated carbon / ethyl acetate; water / 25 - 30 °C / 3677.86 Torr 3.2: 0.5 h / 20 - 25 °C 3.3: 12.5 h / 25 - 30 °C 4.1: sodium hydroxide / dichloromethane; water / 0.5 h / pH 9 - 12 5.1: acetone; methanol / 15 h / -20 - 25 °C 6.1: sodium hydroxide / dichloromethane; water / 0.25 h / pH 11 - 14 View Scheme |
-
-
1237496-99-9
6-ethyl-5-fluoropyrimidin-4-yl methanesulfonate

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / dichloromethane / 12 h / 45 - 50 °C 2: lead; zinc; iodine / tetrahydrofuran / -15 - 20 °C / Inert atmosphere 3: ammonium formate / palladium on carbon / toluene / 3 h / 80 °C 4: acetone; methanol / 1 h / Reflux 5: sodium hydrogencarbonate / dichloromethane / 0.5 h View Scheme | |
| Multi-step reaction with 5 steps 1: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / dichloromethane / 12 h / 45 - 50 °C 2: lead; zinc; iodine / tetrahydrofuran / 2 h / -15 - -10 °C / Inert atmosphere 3: ammonium formate / 10% palladium on charcoal / toluene / 3 h / 80 °C 4: acetone; methanol / 1 h / Reflux 5: sodium hydrogencarbonate / dichloromethane / 0.5 h View Scheme |
-
-
150994-82-4
4-((1,3-bis(octanoyloxy)propan-2-yl)oxy)-4-oxobutanoic acid

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 6h; | 100% |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 3h; | 100% |
-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 1h; | 100% |
-
-
56456-50-9
(2-chloro-6-fluorophenyl)methanol

-
-
137234-62-9
voriconazole

-
-
194798-93-1
Bis(2-chloro-6-fluorobenzyl) (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butyl phosphate

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; anhydrous phosphorus trichloride; dihydrogen peroxide In dichloromethane; tert-butyl methyl ether; water | 88% |
-
-
137234-62-9
voriconazole

-
-
1620283-07-9
C16H13F3N6O3

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 20℃; for 3h; | 88% |
| With sulfuric acid; nitric acid at 0 - 20℃; for 3h; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: C9H16ClNO5; voriconazole With potassium iodide In acetonitrile for 15h; Reflux; Stage #2: With hydrogenchloride; water In methanol at 50 - 55℃; for 20h; Stage #3: In methanol; tert-butyl methyl ether at 10 - 65℃; for 18h; | 87% |
-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With nitric acid In methanol; water | 86.62% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 10h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 12h; Reflux; | 80% |
-
-
431894-46-1
chloromethyl 2-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)ethyl carbonate

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| In acetonitrile for 14h; Reflux; | 79% |
-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water | 78.62% |

| Conditions | Yield |
|---|---|
| With bis-[(trifluoroacetoxy)iodo]benzene; 9-mesityl-10-methylacridinium ion In acetonitrile at 20℃; Irradiation; | A 77% B 11% |

-
-
76-05-1
trifluoroacetic acid

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide for 16h; Inert atmosphere; | 73% |

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| With N,N,N,N-tetraethylammonium tetrafluoroborate; trifluoroacetic acid; 9-(2-mesityl)-10-methylacridinium perchlorate In water; acetonitrile at 20℃; for 10h; Inert atmosphere; Schlenk technique; Irradiation; regioselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: C9H17ClN2O4; voriconazole With potassium iodide In acetonitrile for 15h; Reflux; Stage #2: With hydrogenchloride; water In methanol at 50 - 55℃; for 20h; Stage #3: In methanol; tert-butyl methyl ether at 10 - 65℃; for 15h; | 70% |
-
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
-
137234-62-9
voriconazole

-
-
848469-32-9
(2R,3S)-2-(2,4-diflurophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1yl)butan-2-ol R-(-)-10-camphor sulphonate salt

| Conditions | Yield |
|---|---|
| In methanol; acetone at 30 - 50℃; for 1.33333h; Heating / reflux; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: C23H43ClN4O8; voriconazole With potassium iodide In acetonitrile for 15h; Reflux; Stage #2: With hydrogenchloride; water In methanol at 50 - 55℃; for 20h; Stage #3: In methanol; tert-butyl methyl ether at 10 - 65℃; for 18h; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: chloromethyl (2-methoxyethyl)carbamate; voriconazole With potassium iodide In acetonitrile for 15h; Reflux; Stage #2: In methanol; tert-butyl methyl ether at 10 - 65℃; for 20h; | 63% |
-
-
137234-62-9
voriconazole

-
-
98-89-5
Cyclohexanecarboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; cerium(III) chloride heptahydrate; tetrabutyl-ammonium chloride In 2,2,2-trifluoroethanol; water for 17h; Schlenk technique; Inert atmosphere; Electrochemical reaction; Irradiation; | 60% |

-
-
76-05-1
trifluoroacetic acid

-
-
137234-62-9
voriconazole

| Conditions | Yield |
|---|---|
| Stage #1: potassium trifluoro(isopropenyl)borate(1-); voriconazole With manganese triacetate; acetic acid In water at 50℃; for 18h; Inert atmosphere; Stage #2: trifluoroacetic acid In water; acetonitrile Inert atmosphere; | 58% |


| Conditions | Yield |
|---|---|
| In ethanol; water | 58% |
Voriconazole Chemical Properties
Molecular structure of Voriconazole (CAS NO.137234-62-9) is:
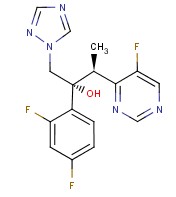
Product Name: Voriconazole
CAS Registry Number: 137234-62-9
IUPAC Name: (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol
Molecular Weight: 349.31047 [g/mol]
Molecular Formula: C16H14F3N5O
XLogP3-AA: 1.5
H-Bond Donor: 1
H-Bond Acceptor: 8
Melting Point: 127-130 °C
Surface Tension: 45.1 dyne/cm
Density: 1.42 g/cm3
Flash Point: 261.4 °C
Enthalpy of Vaporization: 82.02 kJ/mol
Boiling Point: 508.6 °C at 760 mmHg
Vapour Pressure: 3.63E-11 mmHg at 25°C
Product Categories: API; Antifungal (Systemic); Inhibitors; Intermediates & Fine Chemicals; Pharmaceuticals
Voriconazole History
London, UK, 16 December 2008 - The effectiveness of voriconazole in combating fungal infections has been confirmed by a new study to be featured in the International Journal of Antimicrobial Agents ,published by Elsevier.
Voriconazole Uses
Voriconazole (CAS NO.137234-62-9) is generally used to treat serious, invasive fungal infections. These are generally seen in patients who are immunocompromised, and include invasive candidiasis, invasive aspergillosis, and certain emerging fungal infections.
Voriconazole Toxicity Data With Reference
The most common side effects associated with voriconazole(137234-62-9) include transient visual disturbances, fever, rash, vomiting, nausea, diarrhea, headache, sepsis, peripheral edema, abdominal pain, and respiratory disorder.This medication may also cause the skin to peel easily. It is best to apply lotion and/or coconut oil to help with this side effect.
Voriconazole Safety Profile
Safty information about Voriconazole (CAS NO.137234-62-9) is:
Hazard Codes:  Xn
Xn
Risk Statements: 22-36/38
R22:Harmful if swallowed.
R36/38:Irritating to eyes and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
Voriconazole Specification
Voriconazole , its cas register number is 137234-62-9. It also can be called Voriconazole [USAN:INN:BAN] ; (alphaR,betaS)-alpha-(2,4-Difluorophenyl)-5-fluoro-beta-methyl-alpha-(1H-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol ; 4-Pyrimidineethanol, alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1H-1,2,4-triazol-1-ylmethyl)-,(alphaR,betaS)- ; DRG-0301 ; UK-109,496 ; UNII-JFU09I87TR ; VRC ; Vfend ; Voriconazole ; (R-(R*,S*))-alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1H-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol ; 4-Pyrimidineethanol, alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1H-1,2,4-triazol-1-ylmethyl)-, (R-(R*,S*))- . It is a cyrstalline solid.
Related Products
- Voriconazole
- 137234-74-3
- 137234-75-4
- 137234-85-6
- 137234-87-8
- 137234-88-9
- 137234-90-3
- 137235-80-4
- 137245-17-1
- 13725-02-5
- 137254-03-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View