-
Name
(-)-ALPHA-CEDRENE
- EINECS 207-418-4
- CAS No. 469-61-4
- Article Data37
- CAS DataBase
- Density 0.932
- Solubility
- Melting Point 261-262°C
- Formula C15H24
- Boiling Point 261-262 ºC
- Molecular Weight 204.356
- Flash Point 110.2°C
- Transport Information
- Appearance
- Safety A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
- Risk Codes 36/38-50/53
-
Molecular Structure
-
Hazard Symbols


- Synonyms 1H-3a,7-Methanoazulene,2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-, [3R-(3a,3ab,7b,8aa)]-; Cedr-8-ene (8CI); a-Cedrene (6CI,7CI); (-)-Cedrene; (-)-a-Cedrene; Levo-a-cedrene; [3R-(3a,3ab,7b,8aa)]-2,3,4,7,8,8a-Hexahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulene
- PSA 0.00000
- LogP 4.41500
Synthetic route

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile at 50℃; for 0.5h; | 96% |

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid In 1,4-dioxane at 100℃; for 1h; | 93% |
-
-
1262998-09-3
cedrenone

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Stage #1: cedrenone With toluene-4-sulfonic acid hydrazide In ethanol for 2.5h; Caglioti Reductive Elimination; Reflux; Stage #2: With sodium tetrahydroborate In tetrahydrofuran; water for 2h; Reflux; | 92% |
| With potassium hydroxide; hydrazine In diethylene glycol at 125 - 215℃; for 60h; Wolff-Kishner reduction; Inert atmosphere; | 52% |

| Conditions | Yield |
|---|---|
| With acetic acid; hexacarbonyl molybdenum In tetrahydrofuran at 110℃; for 48h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With acetic acid; In 1,4-dioxane at 100℃; for 4h; Product distribution; other tertiary alcohols and acetates; various catalysts and conditions; |
-
-
13567-45-8
9α-hydroxycedrane

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With trichlorophosphate In pyridine; ethanol at 0℃; for 19h; Heating; | 60 mg |
-
-
75-15-0
carbon disulfide

-
-
77-53-2
(+)-cedrol

-
-
74-88-4
methyl iodide

-
A
-
546-28-1
β‐cedrene

-
B
-
469-61-4
alpha-cedrene

-
C
-
35812-25-0
Dithiocarbonic acid S-methyl ester O-((3R,3aS,6R,7R,8aS)-3,6,8,8-tetramethyl-octahydro-3a,7-methano-azulen-6-yl) ester

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| With pyridine; trichlorophosphate In toluene for 16h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With formic acid |

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With ethanol; sodium |

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With formic acid |
-
-
13794-73-5
(3R,3aR,6S,7S,8aS)-3,6,8,8-tetramethylhexahydro-1H-3a,7-cedaran-5(4H)-one

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 2: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |
-
-
13567-40-3
cedran-9-one

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 2 g / 65 percent HClO4 / CH2Cl2 / 24 h / Ambient temperature 2: 400 mg / aq. NaHCO3 / methanol / 3 h / Heating 3: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 4: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |
-
-
74804-69-6, 74804-70-9
cedranone enol benzoate

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 400 mg / aq. NaHCO3 / methanol / 3 h / Heating 2: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 3: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |
-
-
74804-66-3, 74804-67-4
4-desoxo-α-pipitzol benzoate

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Et2O*BF3 / 5 h / Ambient temperature 2: Raney Ni / ethanol / 5 h / Heating 3: 400 mg / aq. NaHCO3 / methanol / 3 h / Heating 4: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 5: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |
-
-
10067-43-3, 10067-44-4
α-pipitzol benzoate

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 13.8 g / Et2O*BF3 / 96 h / Ambient temperature 2: W-7 Raney Ni / ethanol / 5 h / Heating 3: Et2O*BF3 / 5 h / Ambient temperature 4: Raney Ni / ethanol / 5 h / Heating 5: 400 mg / aq. NaHCO3 / methanol / 3 h / Heating 6: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 7: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |
-
-
74804-68-5, 74842-35-6
C24H30O2S2

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Raney Ni / ethanol / 5 h / Heating 2: 400 mg / aq. NaHCO3 / methanol / 3 h / Heating 3: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 4: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |
-
-
74804-65-2, 74841-96-6
C24H28O3S2

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: W-7 Raney Ni / ethanol / 5 h / Heating 2: Et2O*BF3 / 5 h / Ambient temperature 3: Raney Ni / ethanol / 5 h / Heating 4: 400 mg / aq. NaHCO3 / methanol / 3 h / Heating 5: 150 mg / NaBH4 / methanol / 3 h / Ambient temperature 6: 60 mg / POCl3 / pyridine; ethanol / 19 h / 0 °C / Heating View Scheme |

-
A
-
3790-71-4
(2Z,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol

-
B
-
40716-66-3
(E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol

-
C
-
78148-59-1
(-)-(4S,8R)-8-epi-α-bisabolol


-
E
-
1220474-11-2
(+)-2-epi-prezizaene

-
F
-
24048-44-0
(-)-α-acoradiene

-
G
-
469-61-4
alpha-cedrene

-
H
-
28976-67-2
(-)-β-curcumene

| Conditions | Yield |
|---|---|
| With magnesium chloride In pentane at 20℃; for 19h; Inert atmosphere; Enzymatic reaction; | A 6.7 %Spectr. B 3.6 %Spectr. C 1.8 %Spectr. D 1.8 %Spectr. E 44 %Spectr. F 3.9 %Spectr. G 21.5 %Spectr. H 15.5 %Spectr. I 1.3 %Spectr. |

-
-
40716-68-5
(2-cis,6-trans)-farnesyl diphosphate

-
A
-
3790-71-4
(2Z,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol

-
B
-
40716-66-3
(E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol

-
C
-
1220474-11-2
(+)-2-epi-prezizaene

-
D
-
24048-44-0
(-)-α-acoradiene

-
F
-
469-61-4
alpha-cedrene

-
G
-
28976-67-2
(-)-β-curcumene

| Conditions | Yield |
|---|---|
| With tobacco 5-epi-aristolochene synthase Kinetics; Enzymatic reaction; |

-
-
1262789-27-4
C12H16O2

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: n-butyllithium / tetrahydrofuran / 0.25 h / -10 °C / Inert atmosphere 1.2: 1 h / -78 °C / Inert atmosphere 2.1: lead(IV) tetraacetate / 2 h / 20 °C / Inert atmosphere 3.1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 0.02 h / 20 °C / Inert atmosphere 4.1: potassium hydroxide; hydrazine / diethylene glycol / 60 h / 125 - 215 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: n-butyllithium / tetrahydrofuran / 0.25 h / -10 °C / Inert atmosphere 1.2: 1 h / -78 °C / Inert atmosphere 2.1: chloroform / 2 h / -40 - 20 °C / Inert atmosphere 3.1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 0.02 h / 20 °C / Inert atmosphere 4.1: potassium hydroxide; hydrazine / diethylene glycol / 60 h / 125 - 215 °C / Inert atmosphere View Scheme |
-
-
1262789-28-5
C17H24O3

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 0.02 h / 20 °C / Inert atmosphere 2: potassium hydroxide; hydrazine / diethylene glycol / 60 h / 125 - 215 °C / Inert atmosphere View Scheme |
-
-
17194-58-0, 69350-75-0, 70878-71-6, 69301-27-5
2-[(1R)-1,5-dimethylhex-4-enyl]-5-methylphenol

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: lead(IV) tetraacetate / 2 h / 20 °C / Inert atmosphere 2: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 0.02 h / 20 °C / Inert atmosphere 3: potassium hydroxide; hydrazine / diethylene glycol / 60 h / 125 - 215 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: chloroform / 2 h / -40 - 20 °C / Inert atmosphere 2: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 0.02 h / 20 °C / Inert atmosphere 3: potassium hydroxide; hydrazine / diethylene glycol / 60 h / 125 - 215 °C / Inert atmosphere View Scheme |
-
-
372-97-4, 13058-04-3, 25744-33-6, 27248-37-9, 27248-38-0, 40716-68-5
farnesyl pyrophosphate

-
A
-
546-28-1
β‐cedrene

-
B
-
77-53-2
(+)-cedrol

-
C
-
469-61-4
alpha-cedrene

-
D
-
19903-73-2
(-)-(3R,3aS,6S,7R,8aS)-isocedrol

| Conditions | Yield |
|---|---|
| With epicedrol synthase In aq. buffer at 30℃; for 1h; pH=8.5; Enzymatic reaction; |


-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tri-n-butyl-tin hydride / pentane / 0.5 h / 0 °C 2.1: toluene-4-sulfonic acid hydrazide / ethanol / 2.5 h / Reflux 2.2: 2 h / Reflux View Scheme |

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: tert-butyl methyl ether / 6 h / UV-irradiation 1.2: 0.5 h / 0 °C / Inert atmosphere 2.1: tri-n-butyl-tin hydride / pentane / 0.5 h / 0 °C 3.1: toluene-4-sulfonic acid hydrazide / ethanol / 2.5 h / Reflux 3.2: 2 h / Reflux View Scheme |
-
-
469-61-4
alpha-cedrene

-
-
66389-35-3
(8R,9S)-cis-cedrene diol

| Conditions | Yield |
|---|---|
| With pyridine; osmium(VIII) oxide; trimethylamine-N-oxide In water; tert-butyl alcohol for 72h; Heating; | 96% |
| Multi-step reaction with 3 steps 1.1: rose bengal; oxygen / acetonitrile / 4.5 h / 40 °C / Irradiation 1.2: 16 h / 21 °C 2.1: N,N`-sulfuryldiimidazole; dihydrogen peroxide; sodium hydroxide / water; methanol / 5 °C 3.1: lithium aluminium tetrahydride / dichloromethane / 2 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: rose bengal; oxygen / acetonitrile / 4.5 h / 40 °C / Irradiation 1.2: 16 h / 21 °C 2.1: dmap / dichloromethane 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 20 h / 21 °C 4.1: lithium aluminium tetrahydride / 2 h / 0 °C / Inert atmosphere View Scheme | |
| With potassium osmate(VI) dihydrate; 4-methylmorpholine N-oxide In 2-methyl-propan-1-ol; water at 93 - 95℃; for 24h; Solvent; | 115g |
-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With 3,6‐di‐tert‐butyl‐9‐mesityl‐10‐phenylacridin‐10‐ium tetrafluoroborate; ammonium carbonate; 2-amino-benzenethiol In dichloromethane; acetonitrile at 20℃; for 12h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation; | 95% |
| Multi-step reaction with 4 steps 1.1: dimethylsulfide borane complex / tetrahydrofuran / 4 h 1.2: 3 h 2.1: pyridinium chlorochromate / dichloromethane / 3 h / 20 °C 3.1: sodium hydroxide; hydroxylamine hydrochloride / ethanol / 12 h / 20 °C / pH 7 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 12 h / Reflux View Scheme |
-
-
469-61-4
alpha-cedrene

-
-
211379-86-1
(2aR,3R,5aS,7R)-3,6,6,7a-tetramethyloctahydro-2H-2a,7-cedarane[5,6-b]ethylene oxide

| Conditions | Yield |
|---|---|
| With sodium percarbonate; acetic anhydride at 20℃; for 8h; Sonication; | 93% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 25℃; for 8h; | 83.5% |
| With tert.-butylhydroperoxide; di-tert-butyl peroxide In water at 69.84℃; for 8h; Reagent/catalyst; |
-
-
469-61-4
alpha-cedrene

-
-
13567-39-0
9β,10β-epoxy-2,2,6,10-tetramethyltricyclo[5.3.1.03,7]undecane

| Conditions | Yield |
|---|---|
| With monoperoxyphthalic acid In diethyl ether at 5℃; for 96h; | 89% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 0.0333333h; Inert atmosphere; |
-
-
469-61-4
alpha-cedrene

-
-
264881-99-4
methyl 2-(4-bromophenyl)-2-diazoacetate

| Conditions | Yield |
|---|---|
| With dirhodium tetrakis[(R)-(1-(biphenyl)-2,2-diphenylcyclopropanecarboxylate)] In dichloromethane at 22℃; for 3h; Inert atmosphere; Reflux; enantioselective reaction; | 88% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; water at 20℃; for 2h; | 86% |
-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With ruthenium trichloride; sodium periodate In tetrachloromethane; water; acetonitrile at 60℃; for 12h; | 85% |
-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride; tetrabutylammonium nitrate; tetrabutylammonium trifluoromethylsulfonate In dichloromethane at 30℃; for 2.5h; Inert atmosphere; | 85% |
-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| Stage #1: alpha-cedrene With dimethylsulfide borane complex In tetrahydrofuran for 4h; Stage #2: With dihydrogen peroxide; sodium hydroxide In tetrahydrofuran for 3h; | 81% |
-
-
469-61-4
alpha-cedrene

-
A
-
14745-84-7
2-(N,N-dimethylaminomethyl)pyrrole

-
B
-
109-97-7
pyrrole

-
C
-
78307-76-3
N,N-dimethyl-1H-pyrrol-1-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 24h; Heating; | A 5.5% B 41% C 53% |

-
-
469-61-4
alpha-cedrene

-
-
30960-39-5
(3R,3aR,7S,8aS)-3,6,8,8-tetramethyl-1,2,3,7,8,8a-hexahydro-4H-3a,7-cedaran-4-one

| Conditions | Yield |
|---|---|
| With pyridine; tert.-butylhydroperoxide; N-hydroxy-3,4,5,6-tetrachlorophthalimide; lithium perchlorate In acetone Electrochemical reaction; chemoselective reaction; | 53% |
| With tert.-butylhydroperoxide; [bis(acetoxy)iodo]benzene In ethyl acetate at -20 - 20℃; for 11h; | 43% |
-
-
469-61-4
alpha-cedrene

-
A
-
28387-62-4
(3R,3aS,7R,8aS)-3,8,8-trimethyl-2,3,4,7,8,8a-hexahydro-1H-3a,7-cedarane-6-formaldehyde

-
B
-
21441-72-5
((3R,3aS,7R,8aS)-3,8,8-trimethyl-2,3,4,7,8,8a-hexahydro-1H-3a,7-cedaran-6-yl)methanol

| Conditions | Yield |
|---|---|
| With selenium; chloroamine-T In dichloromethane at 20℃; for 36h; | A 21% B 52% |

| Conditions | Yield |
|---|---|
| Stage #1: alpha-cedrene With 9,10-Dicyanoanthracene; oxygen In acetonitrile for 4h; Irradiation; Stage #2: With triphenylphosphine In acetonitrile for 0.5h; | A 30% B 45% |
-
-
469-61-4
alpha-cedrene

-
-
13567-41-4
β-cedren-9-α-ol

| Conditions | Yield |
|---|---|
| Stage #1: alpha-cedrene With oxygen; rose bengal In acetonitrile at 40℃; for 4.5h; Irradiation; Stage #2: With triphenylphosphine In acetonitrile at 21℃; for 16h; | 43% |
| Multi-step reaction with 2 steps 1: rose bengal; oxygen / acetonitrile / 4 h / Irradiation 2: triphenylphosphine / acetonitrile / 1 h View Scheme |
-
-
469-61-4
alpha-cedrene

-
-
108-24-7
acetic anhydride

-
A
-
32388-55-9
(3R-(3α,3aβ,7β,8aα))-1-(2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulen-5-yl)ethan-1-one

-
B
-
87798-28-5
2,3,3,7-tetramethyl-11-methylene-12-oxatetracyclo<6.4.1.02,10.04,8>tridecane

-
C
-
87798-29-6
8-acetyl-11-hydroxy-2,6,6,7-tetramethyltricyclo<5.2.2.01,5>undecane

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at 5℃; for 4.5h; | A 14% B 36% C 19% |

-
-
816444-61-8
N-[(1S)-amino(4-tolyl)oxido-λ4-sulfanylidene]tosylamide

-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With Rh2(S-N-(1,8-naphthoyl)alanine)4; bis(tertbutylcarbonyloxy)iodobenzene In methanol; 1,1,2,2-tetrachloroethane at -78 - -35℃; for 72h; Molecular sieve; Inert atmosphere; regioselective reaction; | 34% |
-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| With phenyl[(propan-2-yl)oxy]silane; iron(III)-acetylacetonate In ethanol; 1,2-dichloro-ethane at 20℃; Inert atmosphere; diastereoselective reaction; | 28% |

| Conditions | Yield |
|---|---|
| With biphenyl; 9,10-Dicyanoanthracene; oxygen In acetonitrile for 20h; Irradiation; | A 15.2% B 15.2% |

| Conditions | Yield |
|---|---|
| (i) CH2Cl2, (ii) aq. KOH; Multistep reaction; |
-
-
469-61-4
alpha-cedrene

| Conditions | Yield |
|---|---|
| (i) O3, CH2Cl2, (ii) aq. H2O2, (iii) Br2, NaOH; Multistep reaction; |
-
-
75-77-4
chloro-trimethyl-silane

-
-
469-61-4
alpha-cedrene

-
-
105642-44-2
Trimethyl-((3R,3aS,7R,8aS)-3,8,8-trimethyl-2,3,4,7,8,8a-hexahydro-1H-3a,7-methano-azulen-6-ylmethyl)-silane

| Conditions | Yield |
|---|---|
| With n-butyllithium; N,N,N,N,-tetramethylethylenediamine 1) 8 h, reflux; Yield given. Multistep reaction; |
-
-
469-61-4
alpha-cedrene

-
-
21441-72-5
((3R,3aS,7R,8aS)-3,8,8-trimethyl-2,3,4,7,8,8a-hexahydro-1H-3a,7-cedaran-6-yl)methanol

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In ethanol for 3h; Heating; Yield given; |
-
-
469-61-4
alpha-cedrene

-
-
13567-54-9
8β-H-cedrane

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In acetic acid |
alpha-Cedrene Chemical Properties
Product Name: (-)-Alpha-cedrene
Synonyms: (3R-(3alpha,3Abeta,7beta,8aalpha))-2,3,4,7,8,8a-Hexahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulene ; 1H-3a,7-methanoazulene, 2,3,4,7,8,8a-Hexahydro-3,6,8,8-tetramethyl-, (3R,3aS,7S,8aS)- ; alpha-Cedrene ; Cedr-8-ene ; Levo-alpha-cedrene
Product Categories: Industrial/Fine Chemicals
CAS NO: 469-61-4
Molecular Formula of alpha-Cedrene (CAS NO.469-61-4) : C15H24
Molecular Weight of alpha-Cedrene (CAS NO.469-61-4) : 204.35
Molecular Structure of alpha-Cedrene (CAS NO.469-61-4) :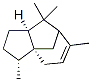
EINECS: 207-418-4
Mol File: 469-61-4.mol
Index of Refraction: 1.515
Surface Tension: 32 dyne/cm
Density: 0.94 g/cm3
Flash Point: 110.2 °C
Enthalpy of Vaporization: 48.01 kJ/mol
Boiling Point: 262.5 °C at 760 mmHg
Vapour Pressure: 0.0177 mmHg at 25°C
Alpha : -88 º(c=10%, EtOH)
alpha-Cedrene Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | FCTXAV Food and Cosmetics Toxicology. 16 (1978),637. |
alpha-Cedrene Consensus Reports
Reported in EPA TSCA Inventory.
alpha-Cedrene Safety Profile
A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes  Xi,
Xi, N
N
Risk Statements 36/38-50/53
R36/38:Irritating to eyes and skin.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements 26-60-61-24/25-23
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S24/25:Avoid contact with skin and eyes.
S23:Do not breathe vapour.
RIDADR UN 3082 9/PG 3
WGK Germany 2
RTECS PB7725000
alpha-Cedrene Specification
Category: Toxic Substances
To stimulate the data: skin - rabbit 500 mg / 24 hours with mild
Flammable hazardous characteristics: thermal decomposition of spicy stimulating smoke
Storage features: low-temperature air drying the Treasury
Fire-extinguishing agent: water, carbon dioxide, foam, dry powder
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View