-
Name
DL-PENICILLAMINE
- EINECS
- CAS No. 52-66-4
- Article Data21
- CAS DataBase
- Density 1.204g/cm3
- Solubility Soluble in ethanol, hot water and sodium hydroxide.
-
Melting Point
204-208 °C(lit.)
- Formula C5H11 N O2 S
- Boiling Point 251.8°Cat760mmHg
- Molecular Weight 149.214
- Flash Point 106.1°C
- Transport Information
- Appearance
- Safety Poison by ingestion and intraperitoneal routes. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and NOx.
- Risk Codes R22;
-
Molecular Structure
-
Hazard Symbols
Xn
- Synonyms DL-Valine,3-mercapto-; Valine, 3-mercapto-, DL- (8CI); (?à)-Penicillamine; 2-Amino-3-mercapto-3-methylbutyricacid; Cysteine, 3,3-dimethyl-; DL-3-Mercaptovaline; DL-Penicillamine; DL-b-Mercaptovaline; DMC; NSC 22880;NSC 44656; b,b-Dimethylcysteine; b-Mercaptovaline; b-Thiovaline
- PSA 102.12000
- LogP 0.80700
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| Behandeln des Reaktionsprodukts mit Essigsaeure und wss.HCl; |
-
-
71-47-6
formate

-
-
15784-30-2
2-amino-2-carboxy-1,1-dimethyl-ethylsulfanyl

-
A
-
52-66-4
DL-Penicillamin

-
B
-
14485-07-5
formate radical

| Conditions | Yield |
|---|---|
| In various solvent(s) at 23℃; Equilibrium constant; pH 6; |

-
-
15784-30-2
2-amino-2-carboxy-1,1-dimethyl-ethylsulfanyl

-
-
3483-12-3, 6892-68-8, 7634-42-6, 16096-97-2, 27565-41-9, 35454-97-8
D,L-dithiothreitol

-
A
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| In water at 23℃; Thermodynamic data; pH=6.0; phosphate buffer; ΔGo; |


| Conditions | Yield |
|---|---|
| In acetonitrile at 37℃; for 72h; Mechanism; Rate constant; pH=2.5; |

-
A
-
52-66-4
DL-Penicillamin

-
B
-
113-79-1, 5591-81-1, 66513-06-2, 66513-07-3, 76023-59-1, 89576-32-9
Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2

| Conditions | Yield |
|---|---|
| In water at 25℃; for 5h; Rate constant; Equilibrium constant; pH 7.0; |


| Conditions | Yield |
|---|---|
| In water at 25℃; for 5h; Rate constant; Equilibrium constant; pH 7.0; |


-
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| With hydrogen bromide | |
| With hydrogenchloride |

-
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminium |

-
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| With ammonia; sodium | |
| With hydrogen bromide |

| Conditions | Yield |
|---|---|
| With phosphonate In water Rate constant; Equilibrium constant; Ambient temperature; |
-
-
67809-83-0
S-nitroso-N-acetylpenicillamine

-
A
-
52-66-4
DL-Penicillamin

-
B
-
312-10-7
DL-penicillamine disulfide

| Conditions | Yield |
|---|---|
| With ethylenediaminetetraacetic acid; buffer reagents; ascorbic acid In water Kinetics; Further Variations:; Reagents; Decomposition; |
-
-
74-93-1
methylthiol

-
-
67809-83-0
S-nitroso-N-acetylpenicillamine

-
A
-
22223-61-6
thionitrous acid S-methyl ester

-
B
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| With ethylenediaminetetraacetic acid In phosphate buffer at 25℃; pH=7.4; Kinetics; Further Variations:; Reaction partners; Reagents; pH-values; concentration; transnitrosation; |

-
-
101115-49-5
N-acetyl-S-benzyl-DL-penicillamine

-
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium; liquid NH3 2: diluted aqueous HCl View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromopent-1-ene; L-penicillamine With cesium hydroxide In dimethyl sulfoxide; N,N-dimethyl-formamide for 12h; Stage #2: With hydrogenchloride In water; dimethyl sulfoxide pH=~ 4 - 5; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 2.5h; pH 9.8; | 100% |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 21h; | 96.4% |
| In methanol at 20℃; | 84% |

| Conditions | Yield |
|---|---|
| In water for 168h; | A n/a B 94% |

-
-
52-66-4
DL-Penicillamin

-
-
2273-45-2
dimethyltin oxide

-
-
74058-30-3, 81021-47-8
chlorodimethyltin(IV) DL-penicillamine

| Conditions | Yield |
|---|---|
| In hydrogenchloride | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 25h; | 90% |
-
-
52-66-4
DL-Penicillamin

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
158944-97-9
2-(tert-butoxycarbonylamino)-3-mercapto-3-methylbutanoic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl-formamide for 16h; | 88% |
| With triethylamine In methanol; ethyl acetate | |
| With potassium hydroxide In tetrahydrofuran | |
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; for 2h; | 2.96 g |
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; for 2h; | 2.96 g |
-
-
37095-27-5
(bis(diphenylphosphino)methane)bis(chlorogold(I))

-
-
52-66-4
DL-Penicillamin

-
-
6018-89-9
nickel(II) acetate tetrahydrate

| Conditions | Yield |
|---|---|
| Stage #1: (bis(diphenylphosphino)methane)bis(chlorogold(I)); DL-Penicillamin With sodium hydroxide In methanol; water Stage #2: nickel(II) acetate tetrahydrate In methanol; water at 20℃; for 1h; | 88% |

-
-
52-66-4
DL-Penicillamin

-
-
99350-06-8
[Au2(1,2-bis(dicyclohexylphosphino)ethane)Cl2]

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 0.166667h; Darkness; | 87% |

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Pb-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 85% |
-
-
52-66-4
DL-Penicillamin

-
-
75-07-0
acetaldehyde

-
-
18455-58-8
2,5,5-trimethyl-1,3-thiazolidine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 83% |

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Pb-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 82% |
-
-
766-40-5
Mucochloric acid

-
-
52-66-4
DL-Penicillamin

-
-
86901-12-4
(3R,7aS)-6,7-Dichloro-2,2-dimethyl-5-oxo-2,3,5,7a-tetrahydro-pyrrolo[2,1-b]thiazole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium chloride In ethanol; acetic acid | 80% |
-
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| With arsenic(III) trioxide In water for 168h; | 80% |
-
-
52-66-4
DL-Penicillamin

-
-
67-64-1
acetone

-
-
58131-62-7
2,2,5,5-tetramethyl thiazolidine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 3.5h; Heating; | 79.5% |
| for 24h; Reflux; | |
| for 1h; Inert atmosphere; Reflux; | 2.6 g |

| Conditions | Yield |
|---|---|
| With acetic anhydride for 3h; Ambient temperature; | 78% |
| With water; acetic anhydride at 60℃; |
-
-
158945-03-0
(+/-)-3-formamido-4,4-dimethylthietan-2-one

-
-
52-66-4
DL-Penicillamin

-
-
158945-04-1
(+/-)-N-(N-formylpenicillaminyl)penicillamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 1.5h; | 77% |

| Conditions | Yield |
|---|---|
| In ethanol; water aminoacid in water was added to the soln. of Me3SnOH in ethanol and stirred for 10 min; evapd. in vac. to dryness, filtered, washed with ethanol, water and diethyl ether, dried; elem. anal.; | 75% |

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Sn-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 75% |

-
-
52-66-4
DL-Penicillamin

-
-
874507-74-1
2,2-dimethyl-5-oxo-thiomorpholine-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium In ammonia; water; acetic acid | 74% |
-
-
52-66-4
DL-Penicillamin

-
-
76-87-9
triphenyltin(IV) hydroxide

-
-
90101-10-3
(C6H5)3SnSC(CH3)2CH(NH2)COOSn(C6H5)3

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Sn-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 72% |
-
-
108865-98-1
α-Acetylamino-β,β-dimethyl-β-propiothiolactone

-
-
52-66-4
DL-Penicillamin

-
-
158944-99-1
(+/-)-N-(N-acetylpenicillaminyl)penicillamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform for 1.5h; | 70% |
-
-
52-66-4
DL-Penicillamin

-
-
122-78-1
phenylacetaldehyde

-
-
89314-95-4
2-benzyl-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 70% |
-
-
82595-77-5
di-μ-chloro-bis[chloro(η5-pentamethylcyclopentadienyl)cobalt]

-
-
52-66-4
DL-Penicillamin

| Conditions | Yield |
|---|---|
| With AgOAc In methanol; water byproducts: AgCl; addn. of organic compound to a soln. of Co-complex in methanol-water, stirring for 24 h at room temperature in the presence of AgOAc; filtn., evapn., dissolving residue in CH2Cl2, layering the soln. with hexane, elem. anal.; | 67% |

| Conditions | Yield |
|---|---|
| In water | 66% |

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Sn-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 65% |

| Conditions | Yield |
|---|---|
| In ethanol | 64% |


| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Sn-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 62% |

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Sn-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 61% |
-
-
52-66-4
DL-Penicillamin

-
-
74-88-4
methyl iodide

-
-
796072-37-2
2-tert-butoxycarbonylamino-3-(4-bromophenylmethylsulfanyl)-3,3-dimethylpropanoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: DL-Penicillamin; 4-bromobenzenemethanol With trifluorormethanesulfonic acid; trifluoroacetic acid In water for 4h; Stage #2: di-tert-butyl dicarbonate With sodium hydroxide In 1,4-dioxane; water at 0℃; for 16h; Stage #3: methyl iodide With hydrogenchloride; potassium carbonate more than 3 stages; | 58% |

| Conditions | Yield |
|---|---|
| In chloroform; water aminoacid in water was added to the soln. of Pb-compd. in CHCl3 and stirred for 15 min; the organic phase dried with Na2SO4, addn. of petroleum ether, evapn. in vac. at 5°C, the ppt. washed with diethyl ether, dried in vac.;elem. anal.; | 58% |
dl-PENICILLAMINE Chemical Properties
IUPAC Name: 2-Amino-3-methyl-3-sulfanylbutanoic acid
The MF of Dl-penicillamine (CAS NO.52-66-4) is C5H11NO2S.
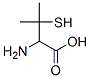
The MW of Dl-penicillamine (CAS NO.52-66-4) is 149.21.
Synonyms of Dl-penicillamine (CAS NO.52-66-4): (2S)-2-Amino-3-methyl-3-sulfanylbutansaure ; 3-Sulfanyl-D-valine ; b,b-Dimethylcysteine ; b-Thiovaline ; Distamine
Product Categories: Amino Acids;Antibiotics;Analytical Chemistry;Biochemistry;Ligands for Pharmaceutical Research;non-Proteinorganic Amino Acids;Radiopharmaceutical Chemistry
Index of Refraction: 1.527
Appearance: white powder
EINECS: 200-147-2
Density: 1.204 g/ml
Flash Point: 106.1 °C
Boiling Point: 251.8 °C
Melting Point: 4-8 °C
storage temp: 2-8 °C
Merck: 14,7088
BRN: 2039817
dl-PENICILLAMINE Uses
Dl-penicillamine (CAS NO.52-66-4) is used in chemical reagents, organic intermediates, fine chemicals, pharmaceutical research and development.
dl-PENICILLAMINE Toxicity Data With Reference
| 1. | pic-esc 100 mg/L | APMBAY Applied Microbiology. 12 (1964),234. | ||
| 2. | orl-rat LD50:365 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 22 (1972),1434. | ||
| 3. | ipr-mus LD50:358 mg/kg | TOLED5 Toxicology Letters. 26 (1985),95. |
dl-PENICILLAMINE Safety Profile
Poison by ingestion and intraperitoneal routes. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and NOx.Safety information of Dl-penicillamine (CAS NO.52-66-4):
Hazard Codes  Xn
Xn
Risk Statements
R36/37/38:Irritating to eyes, respiratory system and skin.
R40:Limited evidence of a carcinogenic effect.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S22:Do not breathe dust.
WGK Germany 3
RTECS YV9445000
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View