-
Name
gamma-Nonanolactone
- EINECS 203-219-1
- CAS No. 104-61-0
- Article Data46
- CAS DataBase
- Density 0.956 g/cm3
- Solubility 9.22g/L(25 oC)
- Melting Point
- Formula C9H16O2
- Boiling Point 266.6 °C at 760 mmHg
- Molecular Weight 156.225
- Flash Point 98.8 °C
- Transport Information
- Appearance
- Safety 24/25-22
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Nonanoic acid, 4-hydroxy-, gamma-lactone;4-Hydroxynonanoicacid lactone;4-Nonanolide;4-Pentyl-butanolide;4-Pentylbutan-4-olide;5-Pentyldihydro-2(3H)-furanone;Cocos aldehyde;Dihydro-5-pentyl-2(3H)-furanone;Nonan-1,4-olide;Prunolide;g-Amyl-g-butyrolactone;g-Amylbutyrolactone;gamma-Pentyl-gamma-butyrolactone;gamma-N-Amylbutyrolactone;
- PSA 26.30000
- LogP 2.27230
Synthetic route

| Conditions | Yield |
|---|---|
| 98% | |
| Electrolysis; | 96% |
| With silica gel; sodium hydrogencarbonate; sodium bromide; 4-hydroxy-TEMPO benzoate In water at 20℃; Electrochemical reaction; | 91% |
| Cp*RuCl(Ph2P(CH2)2NH2-κ2-P,N); potassium tert-butylate In acetone at 30℃; for 1h; | 96 % Spectr. |

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With hydroxide; dihydrogen peroxide | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: acrylic acid methyl ester; hexan-1-ol With Quinuclidine; 2,2-bis(4-(trifluoromethyl)phenyl)-1,3,2λ4-oxazaborolidine; Ir[dF(CF3)ppy]2(4,4′-di-tert-butyl-2,2′-bipyridine)PF6 In acetonitrile at 25 - 33℃; for 14h; Irradiation; Sealed tube; Inert atmosphere; Stage #2: In acetonitrile at 50℃; for 3h; regioselective reaction; | 90% |
| With Quinuclidine; [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; tetrabutylammonium dihydrogen phosphate In acetonitrile at 27℃; Kinetics; Catalytic behavior; Reagent/catalyst; Irradiation; | 84% |
| With di-tert-butyl peroxide; zinc dibromide In water at 180℃; for 12h; Solvent; Time; Dean-Stark; | 70.1% |
| With di-tert-butyl peroxide at 140 - 170℃; Large scale; | 1465 kg |
| With di-tert-butyl peroxide at 20 - 170℃; | 146 g |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 10 - 160℃; | 86.2% |
| With di-tert-butyl peroxide; zinc dibromide at 180℃; for 12h; Dean-Stark; | 76% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; tetraethylammonium tosylate In N,N-dimethyl-formamide Ambient temperature; electroreductive crossed hydrocoupling; | 86% |
-
-
33566-57-3
methyl 4-oxononanoate

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; disodium hydrogenphosphate In methanol for 5h; Ambient temperature; | 85% |
-
-
120040-75-7
γ-(1-Iodo-n-pentyl)-γ-butyrolactone

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In ethanol for 24h; | 85% |

| Conditions | Yield |
|---|---|
| With quinuclidin-3-yl benzenesulfonate; 3,3,3',3'-tetrakis(trifluoromethyl)-1,1'(3H,3'H)-spirobi<2,1-benzoxasilole>; C36H16F16IrN4(1+)*F6P(1-) In acetonitrile at 20℃; for 14h; Glovebox; Inert atmosphere; Sealed tube; Irradiation; | 82% |

| Conditions | Yield |
|---|---|
| 2,2'-azobis(isobutyronitrile) In benzene for 8h; Heating; | 75% |

| Conditions | Yield |
|---|---|
| nickel(II) iodide; samarium diiodide In tetrahydrofuran for 1h; Addition; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: butyl magnesium bromide With copper(I) cyanide In tetrahydrofuran at 0℃; for 0.416667h; Inert atmosphere; Stage #2: methyl 3-(oxiran-2-yl)propanoate In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; diethyl ether; water | 72% |

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In benzene Heating; | 69% |
-
-
35349-81-6
(E)-3-octene-1,1-dicarboxylic acid

-
A
-
35329-50-1
(E)-4-nonenoic acid

-
B
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| at 150 - 160℃; | A 64% B n/a |
-
-
91712-77-5
dihydro-5-(4-iodobutyl)-2(3H)-furanone

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Ambient temperature; | 61% |

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran at 0℃; for 10h; | 57% |
| With samarium diiodide; tert-butyl alcohol In tetrahydrofuran for 3h; Ambient temperature; | 28% |
-
-
66-25-1
hexanal

-
-
140-88-5
ethyl acrylate

-
A
-
57753-68-1
ethyl γ-hydroxypelargonate

-
B
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With borax; ethylenediaminetetraacetic acid; bis(dibutylethyl)hexamethylenediammonium hydroxide; triethylamine In water at 20℃; pH=10; Reagent/catalyst; Solvent; Temperature; Concentration; Electrochemical reaction; | A 48% B 23.7% |

-
-
37651-49-3
diethyl-(2-methoxycarbonyl-ethyl)-methyl-ammonium; iodide

-
-
66-25-1
hexanal

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate In N,N-dimethyl-formamide cathodic reduction; | 47% |
-
-
111861-22-4
5-(2-Hydroxy-heptyl)-2,2-dimethyl-[1,3]dioxane-4,6-dione

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| at 150 - 160℃; for 0.5h; | 41% |
-
-
150171-98-5
(E)-non-3-enenitrile

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
28163-88-4
(E)-non-3-enoic acid

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
70478-77-2
7-hydroxynonanoic acid

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
37056-01-2
4,7-dioxo-nonanoic acid

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; amalgamated zinc; toluene |
-
-
35349-81-6
(E)-3-octene-1,1-dicarboxylic acid

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With sulfuric acid In water for 10h; Heating; |
-
-
127839-79-6
4-Hydroxy-nonanethioic acid benzylamide

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methyl iodide 1.) THF, RT, 2.) 100 deg C, 3 h; Yield given. Multistep reaction; |
-
-
139398-43-9
4-hydroxy-2-nonenoic acid

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 2068.59 Torr; for 15h; Hydrogenation; lactonization; |

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; nickel Hydrogenation.anschliessendes Ansaeuern mit wss. Schwefelsaeure; |

| Conditions | Yield |
|---|---|
| With ethanol; alkaline solution Erhitzen des Reaktionsprodukts unter vermindertem Druck; |

| Conditions | Yield |
|---|---|
| With D-glucose; C. boidinii In phosphate buffer at 20℃; pH=6.0; | A 91 % Chromat. B 4 % Chromat. |
-
-
850480-50-1
4-hydroxynon-2-enal

-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 25 percent / sodium chlorite; 1 mM HCl; sulfamic acid / H2O / 3 h / 20 °C 2: hydrogen / Pd/C / methanol / 15 h / 2068.59 Torr View Scheme |

| Conditions | Yield |
|---|---|
| at 50℃; for 24h; | 98% |
-
-
104-61-0
γ-nonalactone

-
-
57788-65-5
4-hydroxynonanamide

| Conditions | Yield |
|---|---|
| With ammonia at 50 - 60℃; for 168h; | 94% |
-
-
104-61-0
γ-nonalactone

-
-
133287-30-6
(S)-2-amino-3-phenyl-N-[(R)-1-phenylethyl]propanamide

-
-
1219498-04-0
(S)-2-[4-hydroxynonanamide]-3-phenyl-N-[(R)-1-phenylethyl]propanamide

| Conditions | Yield |
|---|---|
| Stage #1: (S)-2-amino-3-phenyl-N-[(R)-1-phenylethyl]propanamide With trimethylaluminum In dichloromethane; toluene at 0℃; for 1h; Stage #2: γ-nonalactone In dichloromethane; toluene at 20℃; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogen; sodium methylate; RuCl2(L-1) In tetrahydrofuran at 100℃; under 37503.8 Torr; for 2.5h; Product distribution / selectivity; | 91% |
| With potassium methanolate; hydrogen; homogeneous ruthenium complex In toluene at 100℃; under 37503.8 Torr; for 4h; | 91% |
| With C15H29MnNO3P2(1+)*Br(1-); potassium tert-butylate; hydrogen In 1,4-dioxane at 110℃; under 22502.3 Torr; for 24h; Inert atmosphere; Autoclave; | 82% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 65℃; for 168h; | 89% |
-
-
104-61-0
γ-nonalactone

| Conditions | Yield |
|---|---|
| With Lawessons reagent; Hexamethyldisiloxane at 120℃; for 0.0416667h; microwave irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In N,N-dimethyl-formamide at 20℃; for 16h; Inert atmosphere; | 83% |
| Stage #1: γ-nonalactone With potassium hydroxide In methanol at 20℃; for 72h; Stage #2: isopropyl bromide In dimethyl sulfoxide at 20℃; for 20h; | 4.62 g |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; methylmagnesium bromide In tetrahydrofuran; diethyl ether at 0 - 20℃; for 1.5h; Inert atmosphere; | 74% |
-
-
760-32-7
diethylmethylsilane

-
-
201230-82-2
carbon monoxide

-
-
104-61-0
γ-nonalactone

-
A
-
5290-28-8
diethylmethylsilyl acetate

-
B
-
104665-10-3
5-<<(diethylmethylsilyl)oxy>-methylene>nonanoic acid diethylmethylsilyl ester

| Conditions | Yield |
|---|---|
| With pyridine; dicobalt octacarbonyl In benzene at 140℃; for 6h; | A n/a B 73% |

-
-
760-32-7
diethylmethylsilane

-
-
201230-82-2
carbon monoxide

-
-
104-61-0
γ-nonalactone

-
-
104665-10-3
5-<<(diethylmethylsilyl)oxy>-methylene>nonanoic acid diethylmethylsilyl ester

| Conditions | Yield |
|---|---|
| With pyridine; dicobalt octacarbonyl In benzene at 140℃; under 38000 Torr; for 6h; | 73% |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol at 50℃; for 5h; | 63% |
-
-
104-61-0
γ-nonalactone

-
-
40657-54-3
dec-5-en-2-one

| Conditions | Yield |
|---|---|
| With acetic acid at 450℃; | 52% |
-
-
50-00-0
formaldehyd

-
-
109-79-5
1-butanethiol

-
-
104-61-0
γ-nonalactone

-
A
-
120388-37-6
5-amyl-5-butylthiotetrahydrofuran-2-one

-
B
-
120388-36-5
5-amyl-3-butylthiomethylenfuran-2-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 45 - 55℃; for 7h; | A 18% B 51% |


| Conditions | Yield |
|---|---|
| pumice; zinc diacetate; manganese(II) acetate at 450℃; | A 50% B 5% |

gamma-Nonanolactone Consensus Reports
COCONUT ALDEHYDE(104-61-0) is reported in EPA TSCA Inventory.
gamma-Nonanolactone Specification
The CAS registry number of gamma-Nonanolactone is 104-61-0. Its EINECS registry number is 203-219-1. The IUPAC name is 5-pentyloxolan-2-one. In addition, the molecular formula is C9H16O2 and the molecular weight is 156.22. It is also called 2(3H)-furanone, dihydro-5-pentyl-. What's more, it belongs to the classes of Carbonyl Compounds; Organic Building Blocks; Lactone Flavors. It should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 1.85; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.85; (4)ACD/LogD (pH 7.4): 1.85; (5)ACD/BCF (pH 5.5): 15.08; (6)ACD/BCF (pH 7.4): 15.08; (7)ACD/KOC (pH 5.5): 242.74; (8)ACD/KOC (pH 7.4): 242.74; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.444; (14)Molar Refractivity: 43.4 cm3; (15)Molar Volume: 163.3 cm3; (16)Polarizability: 17.2 ×10-24cm3; (17)Surface Tension: 30.6 dyne/cm; (18)Density: 0.956 g/cm3; (19)Flash Point: 98.8 °C; (20)Enthalpy of Vaporization: 50.45 kJ/mol; (21)Boiling Point: 266.6 °C at 760 mmHg; (22)Vapour Pressure: 0.00858 mmHg at 25°C.
Preparation of gamma-Nonanolactone: it can be prepared by nonenoic acid through esterification reaction. And then after a series of extraction, washing and distillation you can get the desired product. In addition, it can be prepared by g-Keto-methylnonanoat. This reaction will need reagents NaBH4 and Na2HPO4*12H2O, and solvent methanol. The reaction time is 5 hours with ambient temperature. The yield is about 85%.

Uses of gamma-Nonanolactone: it is used in the preparation of food flavors and feed flavors. In addition, it can react with methylamine to get 4-hydroxy-nonanoic acid methylamide. This reaction needs solvent tetrahydrofuran. The reaction time is 7 days at reaction temperature of 65 °C. The yield is about 89%.
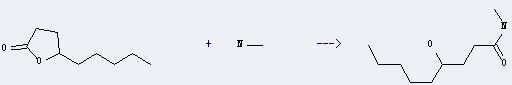
When you are using this chemical, please be cautious about it as the following:
During using it, you should avoid contact with skin and eyes. In addition, do not breathe dust.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1OC(CCCCC)CC1
(2)InChI: InChI=1/C9H16O2/c1-2-3-4-5-8-6-7-9(10)11-8/h8H,2-7H2,1H3
(3)InChIKey: OALYTRUKMRCXNH-UHFFFAOYAG
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 3440mg/kg (3440mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 889, 1975. | |
| rat | LD50 | oral | 6600mg/kg (6600mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 889, 1975. |
Related Products
- gamma-Nonanolactone
- 1046118-40-4
- 104612-36-4
- 104613-64-1
- 104614-74-6
- 104615-18-1
- 1046153-00-7
- 1046153-04-1
- 1046153-20-1
- 104617-49-4
- 104617-51-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View