-
Name
tert-Butyl 2-(4-(pyridin-2-yl)benzyl)hydrazinecarboxylate
- EINECS 631-555-2
- CAS No. 198904-85-7
- Article Data16
- CAS DataBase
- Density 1.117 g/cm3
- Solubility
- Melting Point 74-77 °C
- Formula C17H21N3O2
- Boiling Point
- Molecular Weight 299.373
- Flash Point
- Transport Information
- Appearance white solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-[[4-(2-Pyridinyl)phenyl]methyl]-hydrazinecarboxylic acid 1,1-dimethylethyl ester;
- PSA 63.25000
- LogP 4.05970
Synthetic route
-
-
198904-84-6
2-[4-(2-pyridinyl)phenyl]methylenehydrazinecarboxylic acid tert-butyl ester

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| With hydrogen; sodium carbonate; triethylamine; 5%-palladium/activated carbon at 40℃; under 760.051 Torr; for 8h; | 99.76% |
| With hydrogen; 5%-palladium/activated carbon at 5 - 58℃; under 1520.1 Torr; for 5 - 8h; Nitrogen; | 99.14% |
| With hydrogen; palladium on carbon In methanol under 517.162 Torr; for 4h; | 96.5% |
-
-
198904-84-6
2-[4-(2-pyridinyl)phenyl]methylenehydrazinecarboxylic acid tert-butyl ester

-
A
-
4467-06-5
2-(4-methylphenyl)pyridine

-
B
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| With hydrogen; triethylamine; 5%-palladium/activated carbon at 40℃; under 760.051 Torr; for 5h; | A 0.69% B 99.17% |
| With hydrogen; sodium carbonate; 5%-palladium/activated carbon at 40℃; under 760.051 Torr; for 5h; | A 0.25% B 99.16% |
| With hydrogen; 5%-palladium/activated carbon In methanol at 50℃; under 760.051 Torr; for 5h; | A 2.69% B 97.11% |
| at 40 - 62℃; under 1520.1 Torr; for 5h; Hydrogen/nitrogen (1/2); | A 0.1% B 97.2% |
| With hydrogen; 5%-palladium/activated carbon at 5 - 58℃; under 1520.1 Torr; for 5 - 8h; Nitrogen gas; | A 2.11% B 94.41% |
-
-
857904-11-1
N-(tert-butoxycarbonyl)-N'-[4-(pyridin-2-yl)phenylmethylidene]hydrazine

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In methanol for 4h; | 98% |
| With hydrogen; palladium 10% on activated carbon In methanol | 98% |
| With hydrogen; palladium on activated charcoal In methanol | 89% |
-
-
198904-84-6
2-[4-(2-pyridinyl)phenyl]methylenehydrazinecarboxylic acid tert-butyl ester


-
B
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| palladium-carbon In n-heptane; hydrogen | A n/a B 84% |
-
-
127406-56-8
2-(4-formylphenyl)pyridine

-
-
870-46-2
t-butoxycarbonylhydrazine

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| With borane pyridine; acetic acid In methanol at 0 - 20℃; for 2h; | 78% |
-
-
109-04-6
2-bromo-pyridine

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) Mg, I2, 2.) <1,3-bis(diphenylphosphino)propane>nickel(II) chloride, diisobutylaluminum hydride, 3.) aq. HCl 2: 93 percent / ethanol / 4 h / Heating 3: 89 percent / H2 / 10percent Pd/C / methanol View Scheme | |
| Multi-step reaction with 3 steps 1: tetrakis(triphenylphosphine) palladium(0); sodium carbonate / toluene; ethanol; water / 150 °C / 6750.68 Torr / Sealed tube; Microwave irradiation 2: trimethylsilyl trifluoromethanesulfonate / toluene; isopropyl alcohol / 22 - 50 °C / Microwave irradiation; Sealed tube 3: palladium 10% on activated carbon; sodium formate / ethanol; water / 1.5 h / 57 °C View Scheme | |
| Multi-step reaction with 3 steps 1: tetrakis(triphenylphosphine) palladium(0); sodium carbonate / toluene; ethanol / 20 h / 76 °C / Inert atmosphere 2: toluene; isopropyl alcohol / 2 h / 80 - 85 °C / Inert atmosphere; Large scale 3: palladium 10% on activated carbon; sodium formate / water; ethanol / 1.5 h / 57 °C View Scheme | |
| Multi-step reaction with 3 steps 1: palladium diacetate; triphenylphosphine; potassium hydroxide / methanol; tetrahydrofuran / 24 h / 60 °C / Inert atmosphere; Schlenk technique 2: toluene; isopropyl alcohol / 16 h / 20 - 85 °C / Inert atmosphere; Schlenk technique 3: palladium 10% on activated carbon; sodium formate / ethanol; water View Scheme |
-
-
127406-56-8
2-(4-formylphenyl)pyridine

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93 percent / ethanol / 4 h / Heating 2: 89 percent / H2 / 10percent Pd/C / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: toluene; isopropyl alcohol / 2 h / 80 - 85 °C / Inert atmosphere; Large scale 2: palladium 10% on activated carbon; sodium formate / water; ethanol / 1.5 h / 57 °C View Scheme | |
| Multi-step reaction with 2 steps 1: toluene; isopropyl alcohol / 16 h / 20 - 85 °C / Inert atmosphere; Schlenk technique 2: palladium 10% on activated carbon; sodium formate / ethanol; water View Scheme |
-
-
24856-58-4
1,1-dimethoxy-1-(4-bromophenyl)methane

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) Mg, I2, 2.) <1,3-bis(diphenylphosphino)propane>nickel(II) chloride, diisobutylaluminum hydride, 3.) aq. HCl 2: 93 percent / ethanol / 4 h / Heating 3: 89 percent / H2 / 10percent Pd/C / methanol View Scheme |
-
-
87199-17-5
4-formylphenylboronic acid,

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tetrakis(triphenylphosphine) palladium(0); sodium carbonate / toluene; ethanol; water / 150 °C / 6750.68 Torr / Sealed tube; Microwave irradiation 2: trimethylsilyl trifluoromethanesulfonate / toluene; isopropyl alcohol / 22 - 50 °C / Microwave irradiation; Sealed tube 3: palladium 10% on activated carbon; sodium formate / ethanol; water / 1.5 h / 57 °C View Scheme | |
| Multi-step reaction with 3 steps 1: tetrakis(triphenylphosphine) palladium(0); sodium carbonate / toluene; ethanol / 20 h / 76 °C / Inert atmosphere 2: toluene; isopropyl alcohol / 2 h / 80 - 85 °C / Inert atmosphere; Large scale 3: palladium 10% on activated carbon; sodium formate / water; ethanol / 1.5 h / 57 °C View Scheme | |
| Multi-step reaction with 3 steps 1: palladium diacetate; triphenylphosphine; potassium hydroxide / methanol; tetrahydrofuran / 24 h / 60 °C / Inert atmosphere; Schlenk technique 2: toluene; isopropyl alcohol / 16 h / 20 - 85 °C / Inert atmosphere; Schlenk technique 3: palladium 10% on activated carbon; sodium formate / ethanol; water View Scheme |
-
-
98760-08-8
(2R,3S)-3-[N-(tert-butyloxycarbonyl)amino]-1,2-epoxy-4-phenylbutane

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
198904-86-8
1-[4-(pyridine-2-yl)phenyl]-5(S)-2,5-bis[(tert-butyloxycarbonyl)amino]-4(S)-hydroxy-6-phenyl-2-azahexane

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 24h; Inert atmosphere; Reflux; Large scale; | 85.4% |
| In isopropyl alcohol Heating / reflux; | 75% |
| In isopropyl alcohol for 16h; Heating; | 69% |
-
-
1003888-91-2
C20H32NO7P

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
1003888-92-3
C37H53N4O9P

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 15h; | 78% |
-
-
853893-93-3
(2S)-3-methyl-2-{3-[(2-methyl-1,3-thiazol-4-yl)methyl]-2-oxo-1-imidazolidinyl}butanoic Acid

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; DMF (N,N-dimethyl-formamide) at 25℃; for 16h; | 75% |

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
198905-11-2
1-[4-(Pyridin-2-yl)-phenyl]-4(S)-hydroxy-2-(N-Boc-amino)-5(S)-trifluoroacetyl-amino-6-phenyl-2-azahexane

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 16h; | 60% |
-
-
98760-08-8, 98818-34-9, 98818-35-0, 103127-56-6, 98737-29-2
(1-oxiranyl-2-phenylethyl)carbamic acid tert-butyl ester

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
198904-86-8
1-[4-(pyridine-2-yl)phenyl]-5(S)-2,5-bis[(tert-butyloxycarbonyl)amino]-4(S)-hydroxy-6-phenyl-2-azahexane

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 65℃; for 16h; | 57% |
| In isopropyl alcohol at 65 - 85℃; for 16h; Product distribution / selectivity; | 35% |
| In isopropyl alcohol at 85℃; for 16h; | 35% |
-
-
111398-44-8, 74761-42-5
N-methoxycarbonyl-L-valine

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
857904-23-5
Methyl (1S)-2-methyl-1-({2-[4-(2-pyridinyl)benzyl]hydrazino}carbonyl)propylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine With trifluoroacetic acid In dichloromethane at 25℃; for 1h; Stage #2: N-methoxycarbonyl-L-valine With N-ethyl-N,N-diisopropylamine; 3-[(diethoxyphosphinyl)oxy]-1,2,3-benzotriazin-4(3H)-one In tetrahydrofuran at 25℃; for 3h; | 47% |
| Stage #1: N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine With trifluoroacetic acid In dichloromethane at 25℃; for 1h; Stage #2: N-methoxycarbonyl-L-valine With N-ethyl-N,N-diisopropylamine; 3-[(diethoxyphosphinyl)oxy]-1,2,3-benzotriazin-4(3H)-one In tetrahydrofuran at 25℃; for 3h; | 47% |
-
-
857904-14-4
(1S)-1-(2-oxiranyl)-2-phenyl-N-(trifluoromethyl)ethanamine

-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
857904-15-5
Tert-butyl 2-{(2S,3S)-2-hydroxy-4-phenyl-3-[(trifluoromethyl)amino]butyl}-2-[4-(2-pyridinyl)benzyl]hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 65℃; for 16h; | 28% |
| In isopropyl alcohol at 65℃; for 16h; | 28% |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
137515-66-3
2(R)-3(S)-1,2-epoxy-3-phenylmethoxycarbonylamino-4-phenylbutane

-
-
1346891-05-1
tert-butyl 2-((2S,3S)-2-hydroxy-4-phenyl-3-{[(benzyloxy)carbonyl]amino}butyl)-2-(4-pyridin-2-ylbenzyl)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 18h; Heating / reflux; | 7% |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
1026616-57-8
[ring-13C6]-N-(tert-butoxycarbonyl)-2(S)-amino-1-phenyl-3(R)-3,4-epoxy-bytane

-
-
866017-17-6
[ring-13C6]-1-[4-(pyridin-2-yl)phenyl]-5(S)-2,5-bis[(tert-butyloxy-carbonyl)-amino]-4(S)-hydroxy-6-phenyl-2-azahexane

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 75℃; for 12h; |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
920757-34-2
1-(4-(pyridin-2-yl)benzyl)hydrazine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water at 50℃; for 3h; |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: propan-2-ol / 24 h / 80 °C 2: HCl / dioxane / 4 h / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: propan-2-ol / 24 h / 80 °C 2: HCl / dioxane / 4 h / 50 °C 3: Et3N / tetrahydrofuran / 8 h / 0 - 20 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
437713-06-9
1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxy-5(S)-2,5-diamino-6-phenyl-2-azahexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: propan-2-ol / 24 h / 80 °C 2: HCl / dioxane / 4 h / 50 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCl / tetrahydrofuran; H2O / 3 h / 50 °C 2: 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 / 1.5 h / 20 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
857904-02-0
(S)-{2,2-dimethyl-1-[N'-(4-pyridin-2-yl-benzyl)-hydrazinocarbonyl]-propyl}-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCl / tetrahydrofuran; H2O / 3 h / 50 °C 2: 14.43 g / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 / 1.5 h / 20 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
857900-54-0
{(S)-1-[N'-((2S,3S)-3-amino-2-hydroxy-4-phenyl-butyl)-N'-(4-pyridin-2-yl-benzyl)-hydrazinocarbonyl]-2,2-dimethyl-propyl}-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: HCl / tetrahydrofuran; H2O / 3 h / 50 °C 2: 14.43 g / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 / 1.5 h / 20 °C 3: 25 percent / propan-2-ol / 24 h / Heating 4: HCl / tetrahydrofuran; H2O / 5 h / 50 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
857904-03-1
{(1S,2S)-1-benzyl-2-hydroxy-3-[N'-((S)-2-methoxycarbonylamino-3,3-dimethyl-butyryl)-N-(4-pyridin-2-yl-benzyl)-hydrazino]-propyl}-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: HCl / tetrahydrofuran; H2O / 3 h / 50 °C 2: 14.43 g / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 / 1.5 h / 20 °C 3: 25 percent / propan-2-ol / 24 h / Heating View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: HCl / tetrahydrofuran; H2O / 3 h / 50 °C 2: 14.43 g / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 / 1.5 h / 20 °C 3: 25 percent / propan-2-ol / 24 h / Heating 4: HCl / tetrahydrofuran; H2O / 5 h / 50 °C 5: 78 percent / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / K2HPO4 / CH2Cl2; H2O / 12 h / 20 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

-
-
198904-31-3
atazanavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: HCl / tetrahydrofuran; H2O / 3 h / 50 °C 2: 14.43 g / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 / 1.5 h / 20 °C 3: 25 percent / propan-2-ol / 24 h / Heating 4: HCl / tetrahydrofuran; H2O / 5 h / 50 °C 5: 95 percent / 1-hydroxybenzotriazole; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; ethyldiisopropylamine / CH2Cl2 View Scheme | |
| Multi-step reaction with 3 steps 1: 69 percent / propan-2-ol / 16 h / Heating 2: 100 percent / aq. HCl / tetrahydrofuran / 4 h / 50 °C 3: 1.) O-(1,2-dihydro-2-oxo-1-pyridyl)-N,N,N',N'-tetramethyluronium tetrafluoroborate, N-ethyldiisopropylamine / 1.) CH2Cl2, 0 deg C, 20 min, 2.) CH2Cl2, overnight View Scheme | |
| Multi-step reaction with 3 steps 1: isopropyl alcohol / 24 h / Inert atmosphere; Reflux; Large scale 2: hydrogenchloride / tetrahydrofuran / 3 h / 22 - 55 °C / Large scale 3: N-ethyl-N,N-diisopropylamine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 22 - 25 °C / Large scale View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: propan-2-ol / 12 h / 75 °C 2: HCl / tetrahydrofuran; H2O / 30 h / 60 °C View Scheme |
-
-
198904-85-7
N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridine-2-yl)benzyl]hydrazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: propan-2-ol / 12 h / 75 °C 2: HCl / tetrahydrofuran; H2O / 30 h / 60 °C 3: 0.899 g / 1-hydroxybenzotriazole hydrate; 1-[3-(dimethylamino)-propyl]-3-ethyl-carbodiimide*HCl; diisopropylethylamine / CH2Cl2 / 17 h / 20 °C View Scheme |
tert-Butyl 2-(4-(pyridin-2-yl)benzyl)hydrazinecarboxylate Chemical Properties
Product Name: Tert-butyl N-[(4-pyridin-2-ylphenyl)methylamino]carbamate (CAS NO.198904-85-7)
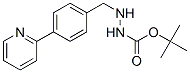
Molecular Formula: C17H21N3O2
Molecular Weight: 299.37g/mol
Mol File: 198904-85-7.mol
Appearance: White Solid
Density: 1.117 g/cm3
Surface Tension: 43.027 dyne/cm
XLogP3-AA: 2.8
H-Bond Donor: 2
H-Bond Acceptor: 4
Structure Descriptors of Tert-butyl N-[(4-pyridin-2-ylphenyl)methylamino]carbamate (CAS NO.198904-85-7):
IUPAC Name: tert-butyl N-[(4-pyridin-2-ylphenyl)methylamino]carbamate
Canonical SMILES: CC(C)(C)OC(=O)NNCC1=CC=C(C=C1)C2=CC=CC=N2
InChI: InChI=1S/C17H21N3O2/c1-17(2,3)22-16(21)20-19-12-13-7-9-14(10-8-13)15-6-4-5-11-18-15/h4-11,19H,12H2,1-3H3,(H,20,21)
InChIKey: GIMJTKJSKPENNG-UHFFFAOYSA-N
Product Categories: API intermediates; Aromatics Compounds; Aromatics; Heterocycles
tert-Butyl 2-(4-(pyridin-2-yl)benzyl)hydrazinecarboxylate Specification
Tert-butyl N-[(4-pyridin-2-ylphenyl)methylamino]carbamate , its CAS NO. is 198904-85-7, the synonyms are N-1-(tert-butoxycarbonyl)-N-2-[4-(pyridin-2-yl)benzyl]hydrazine ; tert-butyl 2-[4-(pyridin-2-yl)benzyl]hydrazinecarboxylate .
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (1-formylcyclopropyl)carbamate
- tert-Butyl (1-hydroxy-3-phenylpropan-2-yl)carbamate
- tert-Butyl (2-chloro-3-formylpyridin-4-yl)carbamate
- tert-Butyl (3S)-3-(hydroxymethyl)morpholine-4-carboxylate
- tert-Butyl (3S)-3-amino-4-phenylbutanoate
- tert-Butyl (3S,4R)-4-(2-methoxyphenyl)pyrrolidin-3-ylcarbamate
- tert-Butyl (3S,4R)-4-phenylpyrrolidin-3-ylcarbamate
- tert-Butyl (4-aminophenyl)carbamate
- tert-Butyl (4-bromothiazol-2-yl)methylcarbamate
- 198904-86-8
- 198904-87-9
- 198907-14-1
- 19892-92-3
- 1989-32-8
- 1989-33-9
- 19893-74-4
- 19893-76-6
- 19894-97-4
- 19894-99-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View