-
Name
tert-Butylamine
- EINECS 200-888-1
- CAS No. 75-64-9
- Article Data165
- CAS DataBase
- Density 0.744 g/cm3
- Solubility miscible with water
- Melting Point -67 °C
- Formula C4H11N
- Boiling Point 44.399 °C at 760 mmHg
- Molecular Weight 73.138
- Flash Point -9 °C
- Transport Information UN 3286 3/PG 2
- Appearance colourless liquid
- Safety 16-26-36/37/39-45-28
- Risk Codes 11-20/22-35-25
-
Molecular Structure
-
Hazard Symbols
 F,
F, C,
C, T
T
- Synonyms tert-Butylamine(8CI);1,1-Dimethylethanamine;1,1-Dimethylethylamine;1-Amino-1,1-dimethylethane;2-Amino-2-methylpropane;2-Aminoisobutane;2-Methyl-2-aminopropane;2-Methyl-2-propanamine;2-Methyl-2-propylamine;Erbumine;N-tert-Butylamine;NSC 9571;Trimethylaminomethane;t-Butylamine;
- PSA 26.02000
- LogP 1.44390
Synthetic route
-
-
4262-38-8
1,3,5,7-Tetra-tert-butyl-2,4,6,8-tetrachloro-[1,3,5,7,2,4,6,8]tetrazatetraborocane

-
A
-
11113-50-1
boric acid

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With water In further solvent(s) byproducts: HCl; solvent cyclohexanol, in tube 20.5 h at 160°C; | A n/a B 97% |
| With H2O In further solvent(s) byproducts: HCl; solvent cyclohexanol, in tube 20.5 h at 160°C; | A n/a B 97% |
| With water In xylene byproducts: HCl; in tube 20.5 h boiling; | A n/a B 62% |
| With H2O In xylene byproducts: HCl; in tube 20.5 h boiling; | A n/a B 62% |

| Conditions | Yield |
|---|---|
| With [Zn(BH4)2(py)] In tetrahydrofuran for 0.67h; Heating; | 96% |
| Stage #1: 2-Methyl-2-nitropropane In water; acetonitrile at 20℃; for 0.0833333h; Stage #2: With sodium tetrahydroborate In water; acetonitrile at 20℃; for 0.5h; | 90% |
| With palladium on activated charcoal; tetrabutylammomium bromide; water; sodium hydroxide; silicon at 100℃; for 6h; | 86% |

| Conditions | Yield |
|---|---|
| With calcium hydroxide at 240 - 270℃; for 1.5h; other hydroxides as reagent;; | 96% |
| With sodium hydroxide In 2-ethoxy-ethanol; water at 100℃; Kinetics; Rate constant; other temperature; activaction energy; | 93% |
| Hydrolysis; | 83% |
| With sodium hydroxide; ethylene glycol | |
| With 2-ethoxy-ethanol; sodium hydroxide |
-
-
111-86-4
n-Octylamine

-
-
28227-42-1, 30834-74-3
1,4-di-tert-butyl-1,4-diazabutadiene

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With formic acid In water; acetonitrile at 15℃; for 30h; | A 92% B n/a |


| Conditions | Yield |
|---|---|
| at 200 - 230℃; for 0.0833333h; Product distribution; pyrolysis without solvent, product isolated as hydrochloride; | A n/a B 86% |
-
-
28227-42-1, 30834-74-3
1,4-di-tert-butyl-1,4-diazabutadiene

-
-
613-73-0
1,2-phenylenediacetonitrile

-
A
-
3029-30-9
naphthalene-1,4-dicarbonitrile

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120℃; for 1h; | A 82% B n/a |


| Conditions | Yield |
|---|---|
| With 5-methyl-1,3,4-thiadiazol-2-amine; triethylamine In ethanol; water at 25℃; for 1h; | 81% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A 80% B n/a C n/a |

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
156-87-6
propan-1-ol-3-amine

-
A
-
5638-63-1
5,6-dihydro-4H-[1,3]oxazine

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With silver cyanide at 90℃; for 18h; | A 70% B n/a |

-
-
125454-66-2
N-(3-phenylprop-2-yn-1-ylidene)-t-butylamine

-
-
1192-72-9
4,5-Dimethyl-2(2H)-imidazolinethione

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-(3-phenylprop-2-yn-1-ylidene)-t-butylamine; 4,5-Dimethyl-2(2H)-imidazolinethione In methanol at 60℃; for 0.5h; Stage #2: With water In methanol | A 70% B n/a |

-
-
22975-87-7
tert-butyl-(di-tert-butyl-diaziridin-3-ylidene)-amine

-
A
-
683223-12-3
1,2,4-tri(tert-butyl)semicarbazide

-
B
-
13952-69-7
1,2-di-tert-butylhydrazine

-
C
-
75-64-9
tert-butylamine

-
D
-
1609-86-5
Tert-butyl isocyanate

| Conditions | Yield |
|---|---|
| With water In 1,4-dioxane for 2h; Reflux; | A 69% B n/a C n/a D n/a |

-
-
125454-66-2
N-(3-phenylprop-2-yn-1-ylidene)-t-butylamine

-
-
2349-58-8
4,5-diphenyl-1H-imidazole-2-thiol

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-(3-phenylprop-2-yn-1-ylidene)-t-butylamine; 4,5-diphenyl-1H-imidazole-2-thiol In methanol at 60℃; for 0.5h; Stage #2: With water In methanol | A 65% B n/a |

-
-
1118-17-8
3-tert-Butylamino-3-methyl-1-butyne

-
A
-
2085-66-7
3-tert-Butylamino-tert-pentylamine

-
B
-
40137-02-8
3-tert-Butylamino-3-methyl-1-butene

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With hydrogen; Lindlar's catalyst In various solvent(s) at -10℃; under 2585.74 Torr; Catalytic hydrogenation; Hydrogenolysis; | A n/a B 64% C n/a |
| With hydrogen; W-2 Raney nickel In ethanol Product distribution; Further Variations:; Catalysts; Solvents; Catalytic hydrogenation; Hydrogenolysis; |


-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
340258-79-9
N-(pyridin-2-yl)thiophene-3-carboxamide

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B 63% C n/a |

-
-
28227-42-1, 30834-74-3
1,4-di-tert-butyl-1,4-diazabutadiene

-
-
109-73-9
N-butylamine

-
A
-
24764-88-3
butyl[2-(butylimino)ethylidene]amine

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With formic acid In water; acetonitrile at 15℃; for 2h; | A 62% B n/a |

-
-
1152035-05-6
2-(3-Bromophenyl)-N-(tert-butyl)imidazo[1,2-a]pyridin-3-amine

-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B n/a C 62% |


-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
321943-80-0
3-methyl-N-(pyridin-2-yl)benzamide

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B 61% C n/a |

-
-
518015-60-6
N-tert-butyl-2-p-tolyl-1H-imidazo[1,2-a]pyridine-3-amine

-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
14547-80-9
4-methyl-N-(pyridine-2-yl)benzamide

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B 60% C n/a |

-
-
857671-17-1
N-tert-butyl-2-(3-methoxyphenyl)imidazo[1,2-a]pyridin-3-amine

-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
114570-45-5
2-methoxy-N-(pyridin-2-yl)benzamide

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B 59% C n/a |


-
A
-
99984-52-8
5-methyl-N-(pyridin-2-yl)furan-2-carboxamide

-
B
-
5336-24-3
1,3-di-tert-butylurea

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A 59% B n/a C n/a |

-
-
853706-94-2
N-(tert-butyl)-2-(2-chlorophenyl)imidazo[1,2-a]pyridin-3-amine

-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
54979-78-1
2-chloro-N-(pyridine-2-yl)benzamide

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B 58% C n/a |


-
A
-
13606-94-5
N-(pyridine-2-yl)propionamide

-
B
-
5336-24-3
1,3-di-tert-butylurea

-
C
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A 57% B n/a C n/a |

-
-
1171753-50-6
N-tert-butyl-2-(3-nitrophenyl)imidazo[1,2-a]pyridin-3-amine

-
A
-
5336-24-3
1,3-di-tert-butylurea

-
B
-
75-64-9
tert-butylamine

-
C
-
85367-01-7
3-nitro-N-(pyridin-2-yl)benzamide

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In m-xylene for 8h; Schlenk technique; Heating; chemoselective reaction; | A n/a B n/a C 56% |

-
-
125454-66-2
N-(3-phenylprop-2-yn-1-ylidene)-t-butylamine

-
-
6857-34-7
2-mercapto-4(5)-phenylimidazole

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-(3-phenylprop-2-yn-1-ylidene)-t-butylamine; 2-mercapto-4(5)-phenylimidazole In methanol at 60℃; for 0.5h; Stage #2: With water In methanol | A 50% B n/a |

-
-
105887-09-0
(E)-3-Phenyl-acrylic acid 1-tert-butylcarbamoyl-1-methyl-2-oxo-propyl ester

-
A
-
105887-13-6
N-tert.butyl 2-acetyl 2-hydroxy propanamide

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; cesium fluoride In tetrahydrofuran for 72h; Heating; | A 45% B n/a |

| Conditions | Yield |
|---|---|
| With ammonia; zeolite BEA (Si/Al ratio of 12.5) at 250℃; under 22502.3 Torr; Product distribution / selectivity; | 44.78% |
| With ammonia; MF114 zeolite at 274.9℃; under 30002.4 Torr; | 8.9% |
| With ammonia; sodium at 250℃; under 661957 Torr; |

| Conditions | Yield |
|---|---|
| Stage #1: N-tert-butylbenzylamine With rhodium(III) tetra(p-sulfonato-phenyl)porphyrin; oxygen; benzaldehyde In methanol; water at 100℃; under 750.075 Torr; for 6h; Stage #2: With hydrogenchloride In methanol; water at 80℃; Catalytic behavior; | 24.5% |

| Conditions | Yield |
|---|---|
| Stage #1: N-isopropyl-N-tert-butylamine With rhodium(III) tetra(p-sulfonato-phenyl)porphyrin; oxygen; benzaldehyde In methanol; water at 100℃; under 750.075 Torr; for 6h; Stage #2: With hydrogenchloride In methanol; water at 80℃; Catalytic behavior; | 22% |
-
-
10375-18-5
Bis-tert.-butylamino-phenylboran

-
A
-
118331-74-1
(t-butylimino)phenylborane

-
B
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| A n/a B 12% | |
| A n/a B 12% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 18h; Molecular sieve; | 100% |
| at 20℃; for 2h; | 100% |
| In dichloromethane at 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; Na12[WZn3(H2O)2(ZnW9O34)2] at 75℃; for 7h; | 100% |
| With potassium permanganate In water 1.) 11 deg C -> room temperature, 8 h, 2.) 55 deg C, 18 h; | 78% |
| With sodium permanganate In hexane at 69℃; for 24h; | 76% |
-
-
108-31-6
maleic anhydride

-
-
75-64-9
tert-butylamine

-
-
32350-46-2
(Z)-4-(tert-butylamino)-4-oxo-2-butenoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; | 100% |
| In toluene at 45 - 50℃; for 1h; | 94% |
| In diethyl ether at 20℃; | 89% |
-
-
108-30-5
succinic acid anhydride

-
-
75-64-9
tert-butylamine

-
-
6622-06-6
4-(tert-butylamino)-4-oxobutanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: succinic acid anhydride; tert-butylamine In N,N-dimethyl acetamide at 20℃; for 24h; Stage #2: In N,N-dimethyl acetamide; xylene at 140℃; for 48h; | 100% |
| In dichloromethane at 20℃; for 1h; | 52% |
| Stage #1: succinic acid anhydride; tert-butylamine In dichloromethane at 20℃; for 1h; Stage #2: With sodium hydroxide In water at 20℃; for 2h; Stage #3: With hydrogenchloride In water at 0℃; | 52% |
-
-
100-07-2
4-methoxy-benzoyl chloride

-
-
75-64-9
tert-butylamine

-
-
19486-73-8
N-tert-butyl-4-methoxybenzamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane | 100% |
| With dmap; sodium hydroxide In dichloromethane at 0 - 20℃; | 100% |
| In tetrahydrofuran at 0℃; | 86% |
-
-
4504-12-5
ethyl 5-oxo-2-phenyl-2,5-dihydroisoxazole-4-carboxaldehyde

-
-
75-64-9
tert-butylamine

-
-
31081-02-4
ethyl (N-t-butylcarbamoyl)(N-phenylcarbamoyl)acetate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform at 0℃; | 100% |
| With hydrogenchloride In dichloromethane at 0 - 20℃; for 0.666667h; | 97% |
| With triethylamine In dichloromethane at 20℃; | 95% |
-
-
122-60-1
Phenyl glycidyl ether

-
-
75-64-9
tert-butylamine

-
-
64980-40-1
1-(N-tert-butyl)amino-3-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| In 2,2,2-trifluoroethanol at 20℃; for 6h; regioselective reaction; | 100% |
| With lithium perchlorate In diethyl ether at 20℃; for 0.5h; | 96% |
| In water at 0 - 20℃; | 96% |
-
-
70-34-8
2,4-Dinitrofluorobenzene

-
-
75-64-9
tert-butylamine

-
-
13059-89-7
N-tert-butyl-2,4-dinitro-aniline

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin In benzene at 25℃; Rate constant; other catalysts, other solvents, var. conc. of catalysts; | 100% |
-
-
1122-91-4
4-bromo-benzaldehyde

-
-
75-64-9
tert-butylamine

-
-
62058-76-8
N-(4-bromobenzylidene)-2-methylpropan-2-amine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; | 100% |
| With 4 A molecular sieve for 24h; Ambient temperature; | 97% |
| With titanium tetrachloride In diethyl ether; toluene at 0℃; for 0.25h; Glovebox; Inert atmosphere; Schlenk technique; | 86% |
| at 100℃; Schlenk technique; | |
| In 1,2-dichloro-ethane at 70℃; for 4h; Glovebox; |
-
-
50-00-0
formaldehyd

-
-
60829-54-1
2,2,5,5-tetramethyl-4-nitromethyleneimidazolidine-1-oxyl

-
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| In methanol; water | 100% |

-
-
19519-59-6
2-chloroacetylenephosphonic acid dimethyl ester

-
-
75-64-9
tert-butylamine

-
-
85829-45-4
dimethyl <2-(tert-butylimino)vinyl>phosphonate

| Conditions | Yield |
|---|---|
| 100% | |
| In diethyl ether for 2h; Ambient temperature; | 98% |
| In diethyl ether at -10 - 20℃; | 98% |
| In diethyl ether at -5 - 20℃; for 2h; | 98% |
-
-
35673-10-0
bis(3-(trifluoromethyl)benzenesulfonyl) peroxide

-
-
75-64-9
tert-butylamine

-
A
-
85462-15-3
N-t-Butyl-O-m-trifluoromethylbenzenesulfonylhydroxylamine

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; for 2h; | A 90% B 100% |

-
-
68654-16-0
6-methylhepto-5-enal

-
-
75-64-9
tert-butylamine

-
-
128266-08-0
6-methylhept-5-en-1-al tert-butylimine

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; for 2h; | A 87% B 100% |

-
-
50777-76-9
(2-formylphenyl)(diphenyl)phosphine

-
-
75-64-9
tert-butylamine

-
-
100350-40-1
N-(tert-butyl)-2-[(diphenylphosphino)benzylidene]amine

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane at 20℃; for 2h; Inert atmosphere; | 100% |
| In toluene at 150 - 160℃; for 6h; Inert atmosphere; | 96% |
| With molecular sieve for 2h; Heating; | 93% |
| In toluene at 110℃; for 18h; Inert atmosphere; Molecular sieve; Sealed tube; Schlenk technique; | 73% |
| Heating; |
-
-
80360-20-9
1-(phenylsulfonyl)indole-3-carbaldehyde

-
-
75-64-9
tert-butylamine

-
-
136878-55-2
3-((tert-butylimino)methyl)-1-(phenylsulfonyl)indole

| Conditions | Yield |
|---|---|
| In benzene for 24h; Heating; | 100% |
| In benzene 1.) RT, 1 h, 2.) reflux, 2 h; |
-
-
111252-28-9
N-<2,4,6-tri(tert-butyl)phenyl>phosphenimidous acid 2,6-di-tert-butyl-4-methylphenyl ester

-
-
75-64-9
tert-butylamine

| Conditions | Yield |
|---|---|
| In benzene for 240h; | 100% |
| In diethyl ether at 20℃; | 100 % Spectr. |
-
-
128346-30-5
(2-formylphenyl)butyl tellurium dibromide

-
-
75-64-9
tert-butylamine

-
-
130191-27-4
2-(tert-butyliminomethinyl)phenyltellurenyl bromide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene | 100% |
-
-
125556-28-7
(Z)-6,10-Dimethyl-5,9-undecadienal

-
-
75-64-9
tert-butylamine

-
-
125556-25-4
(Z)-6,10-Dimethyl-5,9-undecadienal tert-butylimine

| Conditions | Yield |
|---|---|
| In diethyl ether 1.) -10 deg C, 30 min, 2.) room temp., 1.5 h; | 100% |
| In diethyl ether at 20℃; for 1.5h; |

| Conditions | Yield |
|---|---|
| 100% |
-
-
133828-22-5
1,2,2,5,5,6,6-heptamethyl-3-methylene-4-piperidone

-
-
75-64-9
tert-butylamine

-
-
134614-31-6
1,2,2,3,3,6,6-heptamethyl-5-tert-butylaminomethyl-4-piperidone

| Conditions | Yield |
|---|---|
| In ethanol at 25℃; for 168h; | 100% |
-
-
73452-24-1
N-(bis-p-methoxyphenylphosphinoyl)-O-methylsulphonylhydroxylamine

-
-
75-64-9
tert-butylamine

-
-
73452-27-4
N',P-bis-p-methoxyphenyl-N-t-butylphosphonic diamide

| Conditions | Yield |
|---|---|
| 100% |
-
-
75-64-9
tert-butylamine

-
-
824-94-2
p-methoxybenzyl chloride

-
-
22675-83-8
N-(p-methoxybenzyl)-N-tert-butylamine

| Conditions | Yield |
|---|---|
| 100% | |
| With sodium carbonate; sodium iodide In N,N-dimethyl-formamide at 65℃; for 4h; Inert atmosphere; | 56% |
| In N,N-dimethyl-formamide for 6h; Yield given; |
-
-
75-64-9
tert-butylamine

-
-
3127-08-0
bis(hydroxymethyl)phenylphosphine

-
-
139604-87-8
1,5-Di(tert-butyl)-3,7-diphenyl-1,5-diaza-3,7-diphosphacyclooctane

| Conditions | Yield |
|---|---|
| In ethanol | 100% |
-
-
75-64-9
tert-butylamine

-
-
1620-98-0
3,5-di-t-butyl-4-hydroxybenzaldehyde

-
-
71530-29-5
4-<(N-tert-butylimino)methyl>-2,6-di-tert-butylphenyl

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 100% |
-
-
75-64-9
tert-butylamine

-
-
161868-74-2
1,1-dichloro-5-aza-2,8-dioxa-1-phosphaV-dibenzo<9,9',11,11'-tetra-tert-butyl>-bicyclo<3.3.0>octadiene

| Conditions | Yield |
|---|---|
| In benzene for 2h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |
| In ethanol at 20℃; Cooling with ice; | 81% |
-
-
75-64-9
tert-butylamine

-
-
221636-53-9
N,N-dimethyl-5,7-bis(trifluoroacetyl)-8-quinolylamine

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 72h; | 100% |
| In acetonitrile at 50℃; for 72h; Substitution; | 100% |

| Conditions | Yield |
|---|---|
| aluminum oxide; zinc(II) oxide at 100℃; for 2h; Condensation; | 100% |
| In water at 20℃; for 1h; Green chemistry; | 93% |
| In water at 20℃; for 1h; Green chemistry; | 93% |
| Stage #1: carbon disulfide; tert-butylamine In water at 35℃; for 1h; Large scale; Stage #2: With dihydrogen peroxide In vaseline at 65℃; for 2h; Temperature; Large scale; |
tert-Butylamine Consensus Reports
tert-Butylamine Standards and Recommendations
DOT Classification: 8; Label: Corrosive, Flammable Liquid (UN 2734); DOT Class: 3; Label: Flammable Liquid, Corrosive (UN 2733)
tert-Butylamine Specification
The tert-Butylamine, with the CAS registry number 75-64-9, is also known as 2-Amino-2-methylpropane. Its EINECS registry number is 200-888-1. This chemical's molecular formula is C4H11N and molecular weight is 73.14. What's more, both its IUPAC name and systematic name are the same which is called 2-Methylpropan-2-amine. Its classification code is Human Data. The production is an organic chemical compound (specifically, an amine) and occurs as a colorless liquid. It is one of the four isomeric amines of butane, the others being n-Butylamine, sec-Butylamine and Isobutylamine. In addition, it should be kept in a cool and ventilated place.
Physical properties about tert-Butylamine are: (1)ACD/LogP: 0.80; (2)# of Rule of 5 Violations: 0; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 1; (8)#H bond donors: 2; (9)#Freely Rotating Bonds: 1; (10)Polar Surface Area: 26.02 Å2; (11)Index of Refraction: 1.405; (12)Molar Refractivity: 24.081 cm3; (13)Molar Volume: 98.288 cm3; (14)Surface Tension: 23.076 dyne/cm; (15) Density: 0.744 g/cm3; (16)Enthalpy of Vaporization: 28.27 kJ/mol; (17)Boiling Point: 44.399 °C at 760 mmHg; (18)Vapour Pressure: 362.95 mmHg at 25 °C.
Preparation of tert-Butylamine: this chemical can be prepared by tert-Butyl-ure. This reaction needs reagent ethylene glycol and solvents H2SO4, HCN.
(CH3)2C=CH2 + HCN + H2SO4 + 2H2O → (CH3)3CNH2 · H2SO4 + HCOOH
(CH3)3CNH2 · H2SO4 + NH3 → (CH3)3CNH2 + NH4HSO4
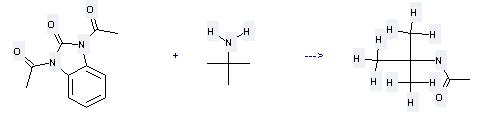
Uses of tert-Butylamine: (1) it is used as an intermediate in the preparation of rubber accelerators, pesticides, pharmaceuticals, dyes and other organic compounds; (2) it is used to produce other chemicals. For example, it is used to produce N-tert-Butyl-acetamide. The reaction occurs with solvent tetrahydrofuran and other condition of heating for 25 hours. The yield is 97 %.
When you are dealing with this chemical, you should be very careful. This chemical may destroy living tissue on contacting. In addition, this chemical is highly flammable and it is harmful by inhalation. What's more, it is toxic if swallowed. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: CC(C)(C)N
(2) InChI: InChI=1S/C4H11N/c1-4(2,3)5/h5H2,1-3H3
(3) InChIKey: YBRBMKDOPFTVDT-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | TCLo | inhalation | 40mg/m3/8H-I (40mg/m3) | SENSE ORGANS AND SPECIAL SENSES: VISUAL FIELD CHANGES: EYE SENSE ORGANS AND SPECIAL SENSES: CORNEAL DAMAGE: EYE SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | British Journal of Industrial Medicine. Vol. 48, Pg. 26, 1991. |
| mouse | LD50 | unreported | 378mg/kg (378mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(12), Pg. 70, 1985. | |
| rabbit | LD50 | unreported | 375mg/kg (375mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(12), Pg. 70, 1985. | |
| rabbit | LDLo | skin | 2gm/kg (2000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: FOOD INTAKE (ANIMAL) GASTROINTESTINAL: OTHER CHANGES | National Technical Information Service. Vol. OTS0570521, |
| rat | LC50 | inhalation | 3800mg/m3/4H (3800mg/m3) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE LUNGS, THORAX, OR RESPIRATION: DYSPNEA BLOOD: HEMORRHAGE | National Technical Information Service. Vol. OTS0570798, |
| rat | LD50 | oral | 44mg/kg (44mg/kg) | BEHAVIORAL: FOOD INTAKE (ANIMAL) LUNGS, THORAX, OR RESPIRATION: PULMONARY EMBOLI LIVER: OTHER CHANGES | National Technical Information Service. Vol. OTS0534846, |
| rat | LD50 | unreported | 450mg/kg (450mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(12), Pg. 70, 1985. |
Related Products
- tert-Butylamine
- tert-Butylamine borane
- 756491-54-0
- 75-65-0
- 756503-69-2
- 7565-14-2
- 756520-67-9
- 756520-78-2
- 75652-49-2
- 756526-01-9
- 75652-74-3
- 75655-44-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View