-
Name
TERT-BUTYLLITHIUM
- EINECS 209-831-5
- CAS No. 594-19-4
- Article Data16
- CAS DataBase
- Density 0.69 g/mL at 20 °C
- Solubility Soluble in n-pentane, soluble in solvents such as hydrocarbon and ethers
- Melting Point
- Formula C4H9Li
- Boiling Point 36-40 °C
- Molecular Weight 64.0565
- Flash Point 20 °F
- Transport Information UN 3394 4.2/PG 1
- Appearance colourless to pale yellow solution
- Safety 26-36/37/39-43-45-62-61-16-33
- Risk Codes 11-15-17-34-65-66-67-50/53-38
-
Molecular Structure
-
Hazard Symbols
 F,
F, C,
C, N
N
- Synonyms Lithium,tert-butyl- (6CI,8CI);(1,1-Dimethylethyl)lithium;t-Butyllithium;tBuLi;(2-Methyl-2-propanyl)lithium;
- PSA 0.00000
- LogP 1.75410
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium In pentane at 20℃; for 2.4h; Product distribution / selectivity; | 35% |
| With lithium In cyclohexane at 40℃; for 4h; Product distribution / selectivity; | 12.7% |
| With lithium; Petroleum ether | |
| With diethyl ether; lithium | |
| With lithium; pentane |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
116178-96-2
1-((R)-Methanesulfinyl)-hex-1-yne

-
-
594-19-4
tert.-butyl lithium

| Conditions | Yield |
|---|---|
| In pentane at -78℃; for 3h; Yield given. Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; |

| Conditions | Yield |
|---|---|
| In hexane at -5℃; | 100% |
| In pentane at 0 - 20℃; Inert atmosphere; | 90% |
| In pentane at -30 - 20℃; for 24h; Inert atmosphere; | 87% |
| In diethyl ether | |
| Inert atmosphere; |
-
-
2401-73-2
1,1,3,3-tetramethyl-1,3-dichlorodisiloxane

-
-
594-19-4
tert.-butyl lithium

-
-
67875-55-2
1,3-bis(1,1-dimethylethyl)-1,1,3,3-tetramethyldisiloxane

| Conditions | Yield |
|---|---|
| In diethyl ether; pentane at -78℃; for 3h; | 100% |
-
-
18187-17-2
2-trimethylsilanyl-acrylic acid

-
-
594-19-4
tert.-butyl lithium

-
-
1115-16-8
4,4-dimethyl-2-(trimethylsilyl)pentanoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; for 3h; | 100% |
-
-
594-19-4
tert.-butyl lithium

-
-
33403-21-3
3,3-ethylendioxy-5α,10α-epoxy-17α-(trimethylsilyloxy)-estr-9(11)-en-17β-carbonitrile

-
-
68027-04-3
3,3-ethylenedioxy-11β-t-butyl-5-hydroxy-17-trimethylsilyloxy-5α-estr-9-ene-17β-carbonitrile

| Conditions | Yield |
|---|---|
| With dimethylsulfide; copper(I) bromide In tetrahydrofuran; hexane 1) -40 deg C, 30 min, 2) -25 deg C, overnight; | 100% |
-
-
594-19-4
tert.-butyl lithium

| Conditions | Yield |
|---|---|
| In diethyl ether; hexane at 0℃; | 100% |
-
-
594-19-4
tert.-butyl lithium

-
-
25979-07-1
tert-butyldichlorophosphine

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In pentane at -80℃; Inert atmosphere; | 100% |
| With phosphorus trichloride In pentane |
-
-
594-19-4
tert.-butyl lithium

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; | 100% |
-
-
594-19-4
tert.-butyl lithium

-
-
77121-88-1
(R)-3,3,3',3'-tetrakis(trifluoromethyl)-1,1'-spirobi<3H,2,1λ5-benzoxaphosphole>

| Conditions | Yield |
|---|---|
| In diethyl ether; pentane at 20℃; for 3h; | 100% |
-
-
1722-12-9
2-chloropyrimidine

-
-
594-19-4
tert.-butyl lithium

-
-
66522-06-3
4-(tert-butyl)-2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In diethyl ether at -30 - 0℃; for 1h; | 100% |
| With acetic acid; bunazosin In tetrahydrofuran at -78℃; for 3h; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; pentane at -40℃; | 100% |
-
-
594-19-4
tert.-butyl lithium

-
-
58963-70-5
trans-3-furan-3-yl-acrylic acid ethyl ester

-
-
147676-16-2
ethyl 4,4-dimethyl-3-(3-furyl)pentanoate

| Conditions | Yield |
|---|---|
| Stage #1: tert.-butyl lithium In diethyl ether; hexane at -78 - -45℃; for 0.75h; complexation; Stage #2: trans-3-furan-3-yl-acrylic acid ethyl ester With chloro-trimethyl-silane In diethyl ether; hexane at -45℃; for 0.5h; Alkylation; Further stages.; | 100% |
-
-
594-19-4
tert.-butyl lithium

-
-
2243-83-6
2-naphthaloyl chloride

-
-
7270-99-7
2,2-dimethyl-1-(naphthalen-2-yl)propan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: tert.-butyl lithium With copper(I) bromide dimethylsulfide complex In tetrahydrofuran; pentane at -50℃; for 0.5h; Stage #2: 2-naphthaloyl chloride In tetrahydrofuran; pentane at -50 - 20℃; | 100% |
-
-
594-19-4
tert.-butyl lithium

-
-
142075-30-7
2-(tert-butylsulfinyl)-N,N-diethylbenzamide

-
-
150079-34-8
2-tert-butyl-N,N-diethylbenzamide

| Conditions | Yield |
|---|---|
| In pentane at -78℃; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; pentane at 0℃; for 1h; Schlenk technique; | 100% |
| In pentane |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane byproducts: NMe3, C4H10; 1.7 M soln. of t-BuLi in hexane was dropped into thf soln. of B-compd. at -78 °C, stirring at this temp. for 1 h, mixt. was brought to room temp. within 5 h; solvent was removed, solid was dried in vac. or 12 h; | 100% |

-
-
439865-84-6
(η5-C5Me5)ZrCl2[N(t-Bu)C(Me)N(Et)]

-
-
594-19-4
tert.-butyl lithium

-
-
590369-38-3
[(η5-C5Me5)ZrCl(iso-butyl)(EtNC(Me)N-t-Bu)]

| Conditions | Yield |
|---|---|
| In diethyl ether at -78°C for 1 h; | 100% |
-
-
1220706-79-5
tert-butyl(1-((3S,6S)-3-((R)-1-iodopropan-2-yl)-6-methyl-cyclohex-1-enyl)vinyloxy)dimethylsilane

-
-
594-19-4
tert.-butyl lithium

-
A
-
1220706-65-9
(S)-6-((1R,4S)-3-(1-(tert-butyldimethylsilyloxy)vinyl)-4-methylcyclohex-2-enyl)hept-2-yn-4-one

-
B
-
71932-99-5
2,2-dimethylhex-4-yn-3-one

| Conditions | Yield |
|---|---|
| In diethyl ether; hexane; pentane at -78℃; Inert atmosphere; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| at -78℃; for 0.25h; Inert atmosphere; Schlenk technique; | 100% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether; pentane at -115℃; for 0.333333h; Inert atmosphere; | 100% |

-
-
594-19-4
tert.-butyl lithium

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; for 0.75h; Inert atmosphere; | 100% |
-
-
594-19-4
tert.-butyl lithium

| Conditions | Yield |
|---|---|
| Stage #1: tert.-butyl lithium With copper(l) cyanide In tetrahydrofuran; pentane at -40℃; for 0.25h; Inert atmosphere; Stage #2: 1,3-diphenyl-1-(pyridin-2-yl)prop-2-yn-1-yl acetate In tetrahydrofuran; pentane at -80 - -10℃; for 4h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 99.3% |
| In tetrahydrofuran; pentane at -78 - 20℃; for 0.166667h; Inert atmosphere; | 99.3% |
-
-
594-19-4
tert.-butyl lithium

-
-
331258-42-5
methyl 7-benzoyl-7-azabicyclo[2.2.1]heptane-1-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; for 2h; | 99% |
-
-
134418-59-0
1,4,8,11-tetraaza-13,13-diethyl-2,2,5,5,7,7,10,10-octamethyl-3,6,9,12,14-pentaoxocyclotetradecane

-
-
594-19-4
tert.-butyl lithium

-
-
7705-08-0
iron(III) chloride

| Conditions | Yield |
|---|---|
| In acetonitrile N2; addn. of 6.8 mmol tert.-BuLi in 2,4-dimethylpentane to a solution of the ligand (1.42 mmol) in CH3CN at -45 ° C; addn. of 2.05 mmol FeCl3; the solution was allowed to warm to room temperature and stirred for 2 h;; precipitation; air admission through a drying tube; filtration; washing with CH2Cl2 and hexanes; drying of the powder under reduced pressure;; | 99% |

-
-
91686-41-8
2,3-bis(trimethylsilyl)-2,3-dicarba-nido-hexaborane(8)

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
594-19-4
tert.-butyl lithium

| Conditions | Yield |
|---|---|
| In pentane; benzene procedure from Organometallics 1993, 12, 3001; addn. t-BuLi (pentane) tosoln. of carborane (C6H6:TMEDA 1:1), stirring (0°C for 2 h, room temp. for 4 h); | 99% |

-
-
874180-89-9
2,4,6-tris[bis(trimethylsilyl)methyl]phenyl mesityl SnF CHC12H8

-
-
594-19-4
tert.-butyl lithium

-
-
874180-87-7
2,4,6-tris[bis(trimethylsilyl)methyl]phenyl mesityl SnCC12H8

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: LiF; Sn complex defluorinated in Et2O with t-BuLi at -40°C, warmed to room temp; LiF removed; | 99% |
-
-
109-99-9
tetrahydrofuran

-
-
132385-06-9
1,1,1-trimethyl-2,2-diphenyldigermane

-
-
594-19-4
tert.-butyl lithium

-
-
936213-06-8
[(1,1-diphenyl-2,2,2-trimethyldigermanyl)lithium(tetrahydrofuran)3]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane Ge-compound in THF treated with t-BuLi in hexane; -10 °C, 1 h; recrystallized from pentane at -15 °C; | 99% |

-
-
172823-06-2
(Sp)-O-(+)-menthyl-H-phosphinate

-
-
594-19-4
tert.-butyl lithium

-
-
82945-11-7, 6057-79-0, 57956-51-1
(Rp)-t-butyl(phenyl)phosphine oxide

| Conditions | Yield |
|---|---|
| In pentane at -80℃; for 17h; Inert atmosphere; optical yield given as %ee; | 99% |
-
-
620-02-0
5-Methylfurfural

-
-
594-19-4
tert.-butyl lithium

-
-
1210782-06-1
2,2-dimethyl-1-(5-methylfuran-2 yl)propan-1-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -10 - 10℃; Inert atmosphere; | 99% |
tert-Butyllithium Specification
The tert-Butyllithium, with the CAS registry number 594-19-4, is also known as (1,1-Dimethylethyl)lithium. It belongs to the product category of Metal Alkyl. Its EINECS number is 209-831-5. This chemical's molecular formula is C4H9Li and molecular weight is 64.06. What's more, its systematic name is (2-Methyl-2-propanyl)lithium. As an organometallic compound, it has applications in organic synthesis since it is a sufficiently strong base to deprotonate many carbon acids, including benzene. tert-Butyllithium is readily available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. tert-Butyllithium is a pyrophoric substance, and it is sensitive to air and water. This chemical can be prepared by halogenated tertiary butane and lithium metal. This reaction will need solvent 1-bromopentane. It is used as catalyst in the catalytic reaction of pharmaceutical intermediates, liquid crystal monomer and organic pesticides.
Uses of tert-Butyllithium: it can be used to produce 1-furan-2-yl-2,2-dimethyl-propan-1-ol at the temperature of 0 °C. It will need solvents diethyl ether, hexane with the reaction time of 1 hour. The yield is about 75%.
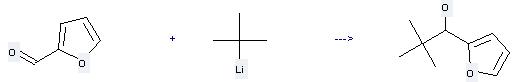
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and is spontaneously flammable in air, so you should keep it away from sources of ignition - No smoking. If contact with water, it will liberate extremely flammable gases. This substance can cause burns and is irritating to skin. Moreover, its vapours may cause drowsiness and dizziness, and repeated exposure may cause skin dryness or cracking.This chemical is very toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. What's more, it is harmful as it may cause lung damage if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing, gloves and eye/face protection. You should take precautionary measures against static discharges. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). In case of fire use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water). You must avoid releasing it to the environment just refering to special instructions/safety data sheet. If swallowed, it will not induce vomiting, but you need to seek medical advice immediately and show this container or label.
You can still convert the following datas into molecular structure:
(1)SMILES: CC(C)(C)[Li]
(2)Std. InChI: InChI=1S/C4H9.Li/c1-4(2)3;/h1-3H3;
(3)Std. InChIKey: BKDLGMUIXWPYGD-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View