-
Name
1,1-DIMETHYL-4-CHLORO-3,5-CYCLOHEXANEDIONE
- EINECS
- CAS No. 7298-89-7
- Article Data28
- CAS DataBase
- Density 1.17g/cm3
- Solubility Insoluble in water.
- Melting Point 161-164 °C
- Formula C8H11ClO2
- Boiling Point 285.4 °C at 760 mmHg
- Molecular Weight 174.627
- Flash Point 119 °C
- Transport Information
- Appearance
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Monochlorodimedone;2-chloro-5,5-dimethyl-cyclohexane-1,3-dione;1,3-Cyclohexanedione, 2-chloro-5,5-dimethyl-;2-Chlorodimedone;Monochlorodimedon;
- PSA 34.14000
- LogP 1.55200
Synthetic route

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane | 95% |
| With N-chloro-succinimide; toluene-4-sulfonic acid In dichloromethane at 0 - 20℃; | 91% |
| With N-chloro-succinimide In various solvent(s) at 20℃; for 0.5h; | 86% |
-
-
126-81-8
dimedone

-
A
-
7298-86-4
2,2-dichloro-5,5-dimethylcyclohexane-1,3-dione

-
B
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With zinc chloride diethyl ether; iron(III)porphyrinate In dichloromethane for 0.5h; | A 20% B 74% |
| With sulfuryl dichloride In benzene at 25℃; for 18h; | A 66% B 26% |
| With potassium bromate; hydrogenchloride In N,N-dimethyl-formamide at 20℃; for 12h; | A 10% B 40% |
| With sodium hypochlorite; iron(III)porphyrinate; triethylbenzylammonium hypochlorite; acetic acid In dichloromethane for 0.5h; Product distribution; various iron(III)porphyrinate adducts, Lewis acids, various products and yields; |
-
-
67-56-1
methanol

-
-
75-09-2
dichloromethane

-
-
1807-68-7
diazodimedone

-
A
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| dirhodium tetraacetate at 18 - 20℃; for 2h; | A 65% B 10% C 6% |


| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate; dichloromethane; saccharin at 0 - 20℃; | 61% |
-
-
126-81-8
dimedone

-
A
-
3359-52-2
bis(4,4-dimethyl-2,6-dioxo-1-cyclohexyl) sulfide

-
B
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With SCl In benzene at 25℃; for 18h; | A 5% B 48% |
-
-
126-81-8
dimedone

-
A
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
B
-
83538-02-7
2-Hydroxydimedon

| Conditions | Yield |
|---|---|
| With 1H-imidazole; citrate-phosphate buffer; Mn-meso-tetrakis(3,5-disulphonatomesityl)porphyrinato; polyvinylpyridinium polymer (MnTMPS/PVP); dihydrogen peroxide; sodium chloride In water; acetonitrile for 1h; Product distribution; Ambient temperature; other reaction partner systems; | A 9% B 26% |
-
-
7298-86-4
2,2-dichloro-5,5-dimethylcyclohexane-1,3-dione

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With acid aqueous solution; potassium iodide | |
| With hydrogenchloride; tin(ll) chloride | |
| With sodium acetate In acetic acid Heating; | |
| Multi-step reaction with 2 steps 1: HCl / dimethylformamide / 0.08 h / 160 - 165 °C 2: NaOAc / acetic acid / Heating View Scheme |
-
-
7298-87-5
2-bromo-2-chloro-5,5-dimethylcyclohexane-1,3-dione

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With potassium hydroxide; urea |
-
-
17554-71-1
2,4-dichloro-5,5-dimethyl-cyclohexane-1,3-dione

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With sodium acetate In acetic acid Heating; |
-
-
126-81-8
dimedone

-
A
-
17530-69-7
3-chloro-5,5-dimethylcyclohex-2-en-1-one

-
B
-
56995-07-4
4',4',6,6-tetramethyl-6,7-dihydro-2'H,6'H-spiro[1,3-benzoxathiol-2,1'-cyclohexan]-2',4,6'(5H)-trion

-
C
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With thionyl chloride In benzene Product distribution; Ambient temperature; investigation of effect of reaction times on product distribution; |

-
-
126-81-8
dimedone

-
A
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
B
-
98593-03-4
2-iodo-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With styrene-co-(4-vinylpyridinium dichloroiodate) (1-) In acetic acid at 50℃; for 5h; Title compound not separated from byproducts; |
-
-
35024-12-5
(4,4-dimethyl-2,6-dioxo-cyclohexyl)-phenyl-iodonium betaine

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With acetyl chloride |
-
-
33932-84-2
2--dimedon-betain

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With hydrogenchloride Heating; |
-
-
23383-02-0
Dimedon-(4-methoxy-phenyl)-iodonium-betain

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
7664-93-9
sulfuric acid

-
-
79255-35-9
2,3-dichloro-5,5-dimethylcyclohex-2-en-1-one

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| at 120℃; |
-
-
7298-86-4
2,2-dichloro-5,5-dimethylcyclohexane-1,3-dione

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
7647-01-0
hydrogenchloride

-
-
7298-86-4
2,2-dichloro-5,5-dimethylcyclohexane-1,3-dione

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
7647-01-0
hydrogenchloride

-
-
7298-86-4
2,2-dichloro-5,5-dimethylcyclohexane-1,3-dione

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
7298-87-5
2-bromo-2-chloro-5,5-dimethylcyclohexane-1,3-dione

-
-
57-13-6
urea

-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: AcCl View Scheme |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

| Conditions | Yield |
|---|---|
| With Oxalyl bromide In tetrahydrofuran at 0 - 20℃; for 2.5h; | 94% |
| With oxalyl bromide In tetrahydrofuran at 0℃; |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
75-04-7
ethylamine

-
-
922-67-8
propynoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ethylamine; propynoic acid methyl ester With iron oxide In neat (no solvent) at 20℃; for 0.5h; Green chemistry; Stage #2: 2-chloro-5,5-dimethyl-1,3-cyclohexanedione In neat (no solvent) for 5h; Green chemistry; | 87% |


| Conditions | Yield |
|---|---|
| With sodium acetate Michael addition; Electrochemical reaction; | 81% |

| Conditions | Yield |
|---|---|
| With sodium acetate Michael addition; Electrochemical reaction; | 80% |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
2393-23-9
4-methoxy-benzylamine

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxy-benzylamine; propynoic acid ethyl ester With iron oxide In neat (no solvent) at 20℃; for 0.5h; Green chemistry; Stage #2: 2-chloro-5,5-dimethyl-1,3-cyclohexanedione In neat (no solvent) for 5h; Green chemistry; | 80% |


| Conditions | Yield |
|---|---|
| With sodium acetate Electrochemical reaction; | 74% |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
7298-86-4
2,2-dichloro-5,5-dimethylcyclohexane-1,3-dione

| Conditions | Yield |
|---|---|
| With iron(III)porphyrinate; boron trifluoride diethyl etherate In dichloromethane for 0.5h; | 73% |
| With sodium hypochlorite; iron(III)porphyrinate; triethylbenzylammonium hypochlorite; acetic acid In dichloromethane for 0.5h; Product distribution; various iron(III)porphyrinate adducts, Lewis acids, various products and yields; | |
| With haloperoxidase from liverwort Bazzania trilobata; potassium chloride; dihydrogen peroxide In phosphate buffer | |
| With vanadium-dependent haloperoxidase from the cyanobacterium Acaryochloris marina; potassium chloride; dihydrogen peroxide In aq. buffer at 30℃; pH=6; Enzymatic reaction; |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
7298-87-5
2-bromo-2-chloro-5,5-dimethylcyclohexane-1,3-dione

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; Pyridine hydrobromide; VOL3(OEt) In chloroform-d1 at 20℃; for 24h; | 70% |
| With chloroform; bromine | |
| With water; bromine | |
| With haloperoxidase from liverwort Bazzania trilobata; dihydrogen peroxide; potassium bromide In phosphate buffer | |
| With vanadate-dependent bromoperoxidase II from Ascophyllum nodosum; dihydrogen peroxide; sodium sulfate; potassium bromide In aq. buffer at 22℃; pH=5.9; Kinetics; pH-value; Enzymatic reaction; |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
120-80-9
benzene-1,2-diol

-
-
63160-43-0
7,8-dihydroxy-3,3-dimethyl-3,4-dihydrodibenzo[b,d]furan-1(2H)-one

| Conditions | Yield |
|---|---|
| With sodium acetate Michael addition; Electrochemical reaction; | 66% |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
A
-
877-08-7
1,2,3,4-tetrachloro-5,6-dimethyl-benzene

-
B
-
79255-35-9
2,3-dichloro-5,5-dimethylcyclohex-2-en-1-one

| Conditions | Yield |
|---|---|
| With chloroform; phosphorus pentachloride; phosphorus trichloride |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
22748-16-9
4,4-dimethylcyclopent-2-ene-1-one

| Conditions | Yield |
|---|---|
| With zinc(II) oxide at 220℃; |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
79255-35-9
2,3-dichloro-5,5-dimethylcyclohex-2-en-1-one

| Conditions | Yield |
|---|---|
| With chloroform; phosphorus pentachloride; phosphorus trichloride | |
| With oxalyl dichloride In tetrahydrofuran at 0 - 20℃; |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
A
-
79255-35-9
2,3-dichloro-5,5-dimethylcyclohex-2-en-1-one

-
B
-
68266-68-2
1,2,3-trichloro-4,5-dimethyl-benzene

| Conditions | Yield |
|---|---|
| With chloroform; phosphorus pentachloride; phosphorus trichloride |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
146335-36-6
5-Chloro-2,3-dimethylphenol

| Conditions | Yield |
|---|---|
| With chloroform; phosphorus pentachloride |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
122-52-1
triethyl phosphite

-
-
80534-76-5
diethyl 5,5-dimethyl-3-oxocyclohex-1-enyl phosphate

| Conditions | Yield |
|---|---|
| With benzene |
-
-
7298-89-7
2-chloro-5,5-dimethyl-1,3-cyclohexanedione

-
-
21428-78-4
4,6-Dibrom-2-chlor-dimedon

| Conditions | Yield |
|---|---|
| With hydrogen bromide; bromine In acetic acid |
Chlorodimedone Specification
The Chlorodimedone, with the CAS registry number 7298-89-7, is also known as 1,3-Cyclohexanedione, 2-chloro-5,5-dimethyl-. This chemical's molecular formula is C8H11ClO2 and molecular weight is 174.62. What's more, its systematic name is 2-Chloro-5,5-dimethylcyclohexane-1,3-dione.
Physical properties of Chlorodimedone are: (1)ACD/LogP: 0.41; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.4; (4)ACD/LogD (pH 7.4): -2.07; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 34.14 Å2; (13)Index of Refraction: 1.476; (14)Molar Refractivity: 42.08 cm3; (15)Molar Volume: 149.1 cm3; (16)Polarizability: 16.68×10-24cm3; (17)Surface Tension: 35.5 dyne/cm; (18)Density: 1.17 g/cm3; (19)Flash Point: 119 °C; (20)Enthalpy of Vaporization: 52.44 kJ/mol; (21)Boiling Point: 285.4 °C at 760 mmHg; (22)Vapour Pressure: 0.00281 mmHg at 25°.
Preparation: this chemical can be prepared by 5,5-Dimethyl-cyclohexane-1,3-dione at the ambient temperature. This reaction will need reagent KIO3 and ClSiMe3 and solvent dimethylformamide with the reaction time of 6 hours. The yield is about 82%.
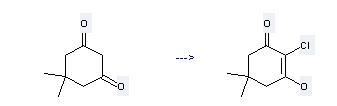
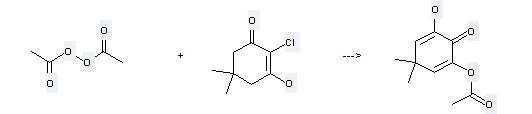
Uses of Chlorodimedone: it can be used to produce 2-Acetoxy-6-hydroxy-4,4-dimethyl-2,5-cyclohexadien-1-one. It will need reagent NaH and solvent dimethylformamide. The yield is about 79%.
When you are using this chemical, please be cautious about it as the following:
Do not breathe dust and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1C(Cl)C(=O)CC(C)(C)C1
(2)Std. InChI: InChI=1S/C8H11ClO2/c1-8(2)3-5(10)7(9)6(11)4-8/h7H,3-4H2,1-2H3
(3)Std. InChIKey: VOBIHUAWDXUCPH-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View