-
Name
Chlorodimethylsilane
- EINECS 213-912-0
- CAS No. 1066-35-9
- Article Data76
- CAS DataBase
- Density 0.852 g/mL at 25 °C(lit.)
- Solubility react with water
- Melting Point -111 °C(lit.)
- Formula C2H7ClSi
- Boiling Point 33.1 °C at 760 mmHg
- Molecular Weight 94.6161
- Flash Point -28 °C
- Transport Information UN 2988 4.3/PG 1
- Appearance Colorless clear liquid
- Safety 16-26-36/37/39-45-9
- Risk Codes 11-34-12
-
Molecular Structure
-
Hazard Symbols
 F,
F,  C,
C,  F+
F+
- Synonyms Chlorodimethylhydrosilane;Chlorodimethylsilane;Dimethylchlorohydrosilane;Dimethylchlorosilane;Dimethylhydrosilyl chloride;Dimethylmonochlorosilane;Dimethylsilyl chloride;SID 4070.0;
- PSA 0.00000
- LogP 1.20860
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether at 60℃; for 0.5h; Temperature; Solvent; | 100% |
| With hydrogenchloride In diethylene glycol dimethyl ether at -45 - 15℃; for 12h; | 99% |

| Conditions | Yield |
|---|---|
| 97% |

| Conditions | Yield |
|---|---|
| With N,N-diethylaminodimethylsilane Heating; | 87% |
| With sodium tetrahydroborate; tetra-n-butylphosphonium chloride In diethylene glycol dimethyl ether at 80 - 140℃; for 26h; | 11% |
| With Dichloromethylsilane; homogeneous catalyst, preferably pyridinium, phosphonium or imidazolium salt Industry scale; |
-
-
814-98-2
1,1,2,2-tetramethyldisilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
1111-74-6
dimethylsilane

-
D
-
993-07-7
trimethylsilan

| Conditions | Yield |
|---|---|
| With tetra-n-butylphosphonium chloride at 180℃; for 17h; Sealed tube; | A 2% B 4% C 87% D 7% |

-
-
15933-59-2
1,1,3,3-tetramethyldisilazane

-
A
-
1066-35-9
dimethylmonochlorosilane

-
B
-
135764-59-9
1,1-dimethyl-3,3,3-trichlorosilazane

-
C
-
135764-60-2
1,1,3,3-tetramethyl-(2-trichlorosilyl)disilazane

| Conditions | Yield |
|---|---|
| With tetrachlorosilane at 20℃; for 24h; | A n/a B n/a C 78% |
| With tetrachlorosilane at 20℃; for 24h; | A n/a B 54% C n/a |

-
-
3277-26-7
1,1,3,3-Tetramethyldisiloxane

-
-
75-78-5
dimethylsilicon dichloride

-
-
1066-35-9
dimethylmonochlorosilane

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In water | 74.9% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; N,N,N,N,N,N-hexamethylphosphoric triamide in a glass ampoule under hermetic conditions; | A 62% B 6.1 % Chromat. |
| With Tetraethylene glycol dimethyl ether; tetra-n-butylphosphonium chloride; lithium hydride In 1,4-dioxane at -196 - 120℃; for 115.5h; Time; Temperature; Reagent/catalyst; Sealed tube; | A 57% B 35% |
| With lithium hydride; triphenylphosphine In diethylene glycol dimethyl ether at -196 - 160℃; for 62h; Time; Reagent/catalyst; Sealed tube; | A 56% B 11% |
-
-
15933-59-2
1,1,3,3-tetramethyldisilazane

-
-
1558-33-4
chloromethylmethyldichlorosilane

-
A
-
1066-35-9
dimethylmonochlorosilane

-
B
-
135790-73-7
1-Chloro-1-chloromethyl-1,3,3-trimethyl-disilazane

| Conditions | Yield |
|---|---|
| at 20℃; for 24h; | A n/a B 61% |

-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
3277-26-7
1,1,3,3-Tetramethyldisiloxane

-
-
75-78-5
dimethylsilicon dichloride

-
-
1066-35-9
dimethylmonochlorosilane

| Conditions | Yield |
|---|---|
| In water | 60% |

-
-
60-29-7
diethyl ether

-
-
1111-74-6
dimethylsilane

-
A
-
1066-35-9
dimethylmonochlorosilane

-
B
-
1825-69-0
dimethyl chloro ethoxy silane

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; for 158h; | A 60% B 26% |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With 2-methylimidazole; tetra-n-butylphosphonium chloride at 175℃; for 89h; Temperature; Reagent/catalyst; Time; Sealed tube; | A 7% B 34% C 57% |
| With tetra-n-butylphosphonium chloride at 140℃; for 64h; Temperature; Schlenk technique; Inert atmosphere; Sealed tube; |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
812-36-2
1,3-dichloro-1,1,2,2,3,3-hexamethyltrisilane

-
B
-
75-77-4
chloro-trimethyl-silane

-
C
-
1066-35-9
dimethylmonochlorosilane

-
D
-
754-75-6
1,4-dichloropermethyltetrasilane

-
E
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With 2-methylimidazole; tetra-n-butylphosphonium chloride at 175℃; for 2.5h; Temperature; Time; Sealed tube; | A 14% B 7% C 34% D 8% E 57% |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
812-36-2
1,3-dichloro-1,1,2,2,3,3-hexamethyltrisilane

-
B
-
75-77-4
chloro-trimethyl-silane

-
C
-
1066-35-9
dimethylmonochlorosilane

-
D
-
1111-74-6
dimethylsilane

-
E
-
754-75-6
1,4-dichloropermethyltetrasilane

-
F
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With tetra-n-butylphosphonium chloride at 220℃; for 21h; Reagent/catalyst; Time; Sealed tube; | A 6% B 15% C 21% D 1% E 1% F 49% |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
993-07-7
trimethylsilan

-
D
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With tetra-n-butylphosphonium chloride at 220℃; for 24h; Sealed tube; | A 16% B 30% C 5% D 44% |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
-
75-78-5
dimethylsilicon dichloride

-
A
-
1066-35-9
dimethylmonochlorosilane

-
B
-
1111-74-6
dimethylsilane

-
C
-
814-98-2
1,1,2,2-tetramethyldisilane

-
D
-
4455-83-8
1-chloro-2-hydro-1,1,2,2-tetramethyldisilane

| Conditions | Yield |
|---|---|
| With tetra-n-butylphosphonium chloride; lithium hydride In diethylene glycol dimethyl ether at -196 - 160℃; for 35h; Sealed tube; | A 42% B 35% C 6% D 5% |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
1111-74-6
dimethylsilane

-
D
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With 2-methylimidazole at 220℃; for 64h; Time; | A 13% B 37% C 10% D 40% |


| Conditions | Yield |
|---|---|
| With Si-Cu; HCl In neat (no solvent) Si-Cu (10:2) and a mixt. of CH4 and HCl at 350°C;; | A 38.8% B 9.4% |
| With hydrogenchloride; copper; silicon In neat (no solvent) Si-Cu (10:2) and a mixt. of CH4 and HCl at 350°C;; | A 38.8% B 9.4% |
-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
1111-74-6
dimethylsilane

-
D
-
993-07-7
trimethylsilan

-
E
-
4519-04-4
dichloro[(chlorodimethylsilyl)methyl]methylsilane

-
F
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With n-Butyl chloride; tributylphosphine at 220℃; for 70h; Sealed tube; | A 13% B 30% C 2% D 2% E 18% F 35% |

-
-
13683-11-9
trimethylsilyl(dimethylchlorosilyl)methane

-
-
5357-38-0
bis(chlorodimethylsilyl)methane

-
-
4519-04-4
dichloro[(chlorodimethylsilyl)methyl]methylsilane

-
-
4519-03-3
bis(methyldichlorosilyl)methane

-
-
75-78-5
dimethylsilicon dichloride

-
A
-
75-54-7
Dichloromethylsilane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
992-94-9
methylsilane

-
D
-
1111-74-6
dimethylsilane

-
E
-
993-00-0
chloro-methyl-silane

| Conditions | Yield |
|---|---|
| With tetra-n-butylphosphonium chloride; lithium hydride In diethylene glycol dimethyl ether at -196 - 220℃; for 31h; Sealed tube; | A 7% B 34% C 9% D 5% E 7% |

-
-
3277-26-7
1,1,3,3-Tetramethyldisiloxane

-
-
368-44-5
dichloro(fluoro)(phenyl)silane

-
A
-
1066-35-9
dimethylmonochlorosilane

-
B
-
1344680-26-7
phenyl(dimethylsiloxy)fluorochlorosilane

| Conditions | Yield |
|---|---|
| at 20℃; for 14h; | A n/a B 30% |

-
-
75-54-7
Dichloromethylsilane

-
A
-
75-79-6
Methyltrichlorosilane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
993-00-0
chloro-methyl-silane

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 150℃; for 4h; | A 25.1% B 3.5% C 2.9% |
| dihydrogen hexachloroplatinate at 150℃; for 4h; | A 25.1% B 3.5% C 2.9% |

-
-
75-79-6
Methyltrichlorosilane

-
-
75-78-5
dimethylsilicon dichloride

-
A
-
75-54-7
Dichloromethylsilane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
992-94-9
methylsilane

-
D
-
993-00-0
chloro-methyl-silane

| Conditions | Yield |
|---|---|
| With Tetraethylene glycol dimethyl ether; tetra-n-butylphosphonium chloride; lithium hydride at 80 - 120℃; for 86.25h; | A 17% B 19% C 11% D 23% |

-
-
1450-14-2
1,1,1,2,2,2-hexamethyldisilane

-
-
1560-28-7
pentamethylchlorodisilane

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
-
13528-88-6
1,1,2-trichloro-1,2,2-trimethyldisilane

-
-
4518-98-3
1,1,2,2-tetrachloro-1,2-dimethyldisilane

-
-
4518-99-4
1,1-dichlorotetramethyldisilane

-
A
-
75-54-7
Dichloromethylsilane

-
B
-
75-77-4
chloro-trimethyl-silane

-
C
-
75-79-6
Methyltrichlorosilane

-
D
-
1066-35-9
dimethylmonochlorosilane

-
E
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 430 - 750℃; for 72h; Conversion of starting material; | A 4 %Chromat. B 12 %Chromat. C 7 %Chromat. D 6 %Chromat. E 21% |

-
-
812-15-7
1,1,1,2,2-pentamethyldisilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
1066-35-9
dimethylmonochlorosilane

-
C
-
993-07-7
trimethylsilan

-
D
-
1560-28-7
pentamethylchlorodisilane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dibutyl ether; water at 70℃; for 24h; | A 5% B 22.8 %Spectr. C 26 %Spectr. D 6.3 %Spectr. |
| With hydrogenchloride In dibutyl ether; benzene-d6 at 70℃; for 24h; Sealed tube; |

-
-
13465-77-5
hexachlorodisilane

-
-
2304-30-5
tetra-n-butylphosphonium chloride

-
-
1066-35-9
dimethylmonochlorosilane

| Conditions | Yield |
|---|---|
| With 2-methylimidazole at 175℃; for 2.5h; Sealed tube; | 5% |
-
-
34557-54-5
methane

-
-
1066-35-9
dimethylmonochlorosilane

| Conditions | Yield |
|---|---|
| With hydrogenchloride; copper(II) hydroxide; silicon at 350℃; |

| Conditions | Yield |
|---|---|
| at 200℃; |
-
-
14857-34-2
dimethyl(ethoxy)silane

-
-
1719-58-0
Chlorodimethylvinylsilane

-
A
-
1066-35-9
dimethylmonochlorosilane

-
B
-
5356-83-2
ethoxydimethylvinylsilane

| Conditions | Yield |
|---|---|
| at 28.5℃; Equilibrium constant; |


| Conditions | Yield |
|---|---|
| at 90℃; for 5h; Inert atmosphere; | 100% |
| With platinum on carbon nanotubes In neat (no solvent) at 20℃; for 24h; | 97% |
| dihydrogen hexachloroplatinate In diethyl ether for 15h; Heating; | 80% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
1113-12-8
diallyl(dimethyl)silane

-
-
55392-19-3
1-(Chloro-dimethyl-silanyl)-3-{[3-(chloro-dimethyl-silanyl)-propyl]-dimethyl-silanyl}-propane

| Conditions | Yield |
|---|---|
| With platinum 1,3-divinyl-1,1,3,3-tetramethyldisiloxane In 5,5-dimethyl-1,3-cyclohexadiene; hexane at 20℃; Inert atmosphere; Reflux; | 100% |
| platinum on activated charcoal In diethyl ether Heating; |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
7766-49-6
1-iodo-10-undecene

-
-
139764-33-3
11-(dimethylchlorosilyl)-1-iodoundecane

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane for 12h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
110661-49-9
10-undecylenic acid N-hydroxysuccinimide ester

-
-
139764-32-2
N-succinimidyl 11-(dimethylchlorosilyl)undecanoate

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane for 12h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
139764-38-8
2-Methylene-succinic acid 4-(11-bromo-undecyl) ester 1-[11-((S)-2-dimethylamino-3-phenyl-propoxycarbonyl)-undecyl] ester

-
-
139764-39-9
2-(Chloro-dimethyl-silanylmethyl)-succinic acid 4-(11-bromo-undecyl) ester 1-[11-((S)-2-dimethylamino-3-phenyl-propoxycarbonyl)-undecyl] ester

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane for 12h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
95-55-6
2-amino-phenol

-
-
18245-89-1
2,2-dimethyl-2,3-dihydro-benzo[1,3,2]oxazasilole

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 6h; Heating; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
146063-23-2
[Methyl-(2-{tris-[2-(methyl-divinyl-silanyl)-ethyl]-silanyl}-ethyl)-vinyl-silanyl]-ethene

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane (Karstedt's catalyst) In diethyl ether at 50℃; for 20h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
151626-10-7
tetrakis[2-(trivinylsilyl)ethyl]silane

| Conditions | Yield |
|---|---|
| bis(tetrabutylammonium) hexachloroplatinate(IV) In ethanol | 100% |
| chloroplatinic acid 1,1,3,3-tetramethyl-1,3-divinyldisiloxane complex Hydrosilation; | |
| platinum Addition; |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
42403-77-0
(4-(allyloxy)phenyl)(phenyl)methanone

| Conditions | Yield |
|---|---|
| platinum on activated charcoal for 5h; Addition; hydrosilation; Heating; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
18269-92-6
bis-allyloxy-dimethyl-silane

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 25℃; for 3.25h; | 100% |

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 25℃; for 3.25h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
394737-60-1
3-[allyloxy-(3-{[3-(bis-allyloxy-methyl-silanyl)-propoxy]-dimethyl-silanyloxy}-propyl)-methyl-silanyloxy]-propene

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate In diethyl ether at 25℃; for 1.25h; | 100% |

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 25℃; for 3.25h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane


| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 25℃; for 3.25h; | 100% |

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 25℃; for 3.25h; | 100% |

| Conditions | Yield |
|---|---|
| dihydrogen hexachloroplatinate at 25℃; for 3.25h; | 100% |

| Conditions | Yield |
|---|---|
| With bis(tetrabutylammonium) hexachloroplatinate(IV) at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; pentane at -78 - 20℃; | 100% |
| Stage #1: hex-1-yne With ethylmagnesium bromide In tetrahydrofuran; diethyl ether at 0 - 20℃; for 0.0833333h; Inert atmosphere; Stage #2: dimethylmonochlorosilane In tetrahydrofuran; diethyl ether at 0 - 20℃; for 4h; Inert atmosphere; | 98% |
| Stage #1: hex-1-yne With n-butyllithium In diethyl ether; hexane at -78℃; for 0.5h; Stage #2: dimethylmonochlorosilane In diethyl ether; hexane at -78 - 20℃; Further stages.; | 85% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
648930-60-3
4-(pent-4-en-1-yloxy)-1,1'-biphenyl

-
-
761401-74-5
4-biphenyloxypentyldimethylchlorosilane

| Conditions | Yield |
|---|---|
| chloroplatinic acid 1,1,3,3-tetramethyl-1,3-divinyldisiloxane complex In xylene at 60℃; for 24h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
70964-99-7
acetic acid 2-allyloxyethyl ester

| Conditions | Yield |
|---|---|
| divinyltetramethylsiloxaneplatinum(O) In xylene at 30 - 36℃; for 120h; | 100% |
-
-
868152-12-9
N-(10'-undecenyl)-12,14-dinitrodehydroabietamide

-
-
1066-35-9
dimethylmonochlorosilane

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane; isopropyl alcohol Heating; | 100% |
-
-
868152-15-2
4-(10'-undecenylaminocarbonyl)-1-(friedelan-3α-yl)-benzoate

-
-
1066-35-9
dimethylmonochlorosilane

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane; isopropyl alcohol Heating; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
1093380-37-0
Zr[Me2Si(η5-C5Me4)(η5-C5H3CMe2CH2CH2CHCH2)]Cl2

-
-
1093380-62-1
Zr[Me2Si(η5-C5Me4)(η5-C5H3CMe2(CH2)4SiMe2Cl)]Cl2

| Conditions | Yield |
|---|---|
| divinyltetramethylsiloxaneplatinum(O) In toluene (N2), 2 drops of catalyst were added to soln. of Zr complex in toluene, stirred at room temp. for 10 min, treated with excess SiHMe2Cl dropwise,stirred for 6 h; filtered, evapd.(vac.), elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In toluene at 90℃; for 18h; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
129750-23-8
Ethyl 3,4,6-tri-O-benzyl-1-thio-α-D-mannopyranoside

-
-
1180492-12-9
ethyl 2-O-dimethylsilane-3,4,6-tri-O-benzyl-1-thio-α-D-mannopyranoside

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; Inert atmosphere; | 100% |
| With triethylamine |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
180187-58-0
ethyl 3,4,6-tri-O-benzyl-1-thio-β-D-glucopyranoside

-
-
1180492-09-4
ethyl 2-O-dimethylsilane-3,4,6-tri-O-benzyl-1-thio-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; Inert atmosphere; | 100% |
| With triethylamine |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
90-11-9
1-Bromonaphthalene

-
-
38274-80-5
dimethyl(naphthalen-1-yl)silane

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromonaphthalene With diisobutylaluminium hydride; magnesium; lithium chloride In tetrahydrofuran; hexane at 20℃; for 3h; Schlenk technique; Inert atmosphere; Stage #2: dimethylmonochlorosilane In tetrahydrofuran; hexane at 20℃; Schlenk technique; Inert atmosphere; | 100% |
| With n-butyllithium In tetrahydrofuran; hexane at -78 - 20℃; for 1h; Inert atmosphere; | 96.7% |
| Stage #1: 1-Bromonaphthalene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.333333h; Inert atmosphere; Stage #2: dimethylmonochlorosilane In tetrahydrofuran; hexane at -78 - 20℃; for 0.5h; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane for 18h; Reflux; | 100% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
1379460-62-4
C79H132O6

-
-
1379460-56-6
2-(ω-(chlorodimethylsilyl)-undecyl)-3,6,7,10,11-penta-decyloxytriphenylene

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane for 18h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In dichloromethane for 18h; Reflux; | 100% |
Chlorodimethylsilane Specification
The Chlorodimethylsilane with the CAS registry number 1066-35-9, is also known as Dimethylchlorohydrosilane. It belongs to the product categories of Dimethylsilylation (GC Derivatizing Reagents); Analytical Chemistry; GC Derivatizing Reagents; Monochlorosilanes; Reduction; Si (Classes of Silicon Compounds); Si-Cl Compounds; Si-H Compounds;Silylation (GC Derivatizing Reagents); Synthetic Organic Chemistry; Chloro Silanes; Hydrogensilanes Hydrogensiloxanes; Reducing Agents. Its EINECS number is 213-912-0. This chemical's molecular formula is C2H7ClSi and molecular weight is 94.62. What's more, its systematic name is chloro(dimethyl)silane. It is a silane, with a silicon atom bonded to two methyl groups, a chlorine atom, and a hydrogen atom. It is stable at common pressure and temperature, and it should be sealed and stored in containers with dry inert gas. Moreover, it should be protected from oxides, alcohol and water.
Physical properties of Chlorodimethylsilane: (1)ACD/LogP: 2.76; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.76; (4)ACD/LogD (pH 7.4): 2.76; (5)ACD/BCF (pH 5.5): 73.5; (6)ACD/BCF (pH 7.4): 73.5; (7)ACD/KOC (pH 5.5): 754.23; (8)ACD/KOC (pH 7.4): 754.23; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Enthalpy of Vaporization: 26.68 kJ/mol; (13)Boiling Point: 33.1 °C at 760 mmHg; (14)Vapour Pressure: 569 mmHg at 25°C.
Preparation of Chlorodimethylsilane: This chemical can be prepared by silica powder and chloromethane at the temperature of 300 °C. This reaction will also need catalyst copper.
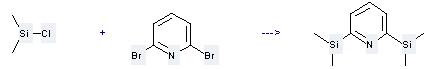
Uses of Chlorodimethylsilane: It can be used to produce 2,6-bis-dimethylsilanyl-pyridine by heating. It will need reagent Mg and solvent tetrahydrofuran with the reaction time of 3 hours. The yield is about 61%.
When you are using this chemical, please be cautious about it as the following:
Chlorodimethylsilane is highly flammable, so you should keep it away from sources of ignition - No smoking. It can cause burn. It is irritating to eyes, respiratory system and skin. You should keep the container in a well-ventilated place. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1S/C2H6ClSi/c1-4(2)3/h1-2H3
(2)InChIKey: QABCGOSYZHCPGN-UHFFFAOYSA-N
(3)Canonical SMILES: C[Si](C)Cl
Related Products
- Chlorodimethylsilane
- 106-63-8
- 1066-40-6
- 1066-45-1
- 106649-02-9
- 106649-95-0
- 106-65-0
- 106650-56-0
- 1066-50-8
- 1066-51-9
- 1066-54-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View