-
Name
Cladribine
- EINECS 224-427-9
- CAS No. 4291-63-8
- Article Data38
- CAS DataBase
- Density 1.402 g/cm3
- Solubility
- Melting Point 181-185 °C(lit.)
- Formula C10H12ClN5O3
- Boiling Point 387.1 °C at 760 mmHg
- Molecular Weight 285.69
- Flash Point 187.9 °C
- Transport Information
- Appearance White Crystalline Solid
- Safety 26-45-36-22
- Risk Codes 36/37/38-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 2-Chloro-2-deoxy-beta-adenosine;2-Chloro-6-amino-9-(2-deoxy-beta-D-erythropentofuranosyl)purine;Chlorodeoxyadenosine;RWJ 26251;Adenosine,2-chloro-2'-deoxy-;Leustatin;24757-90-2;2-CdA;2-Chlorodeoxyadenosine;Adenosine, 2-chloro-2-deoxy;Cladribine(Base);Cadribine;Leustatin (TN);Cladribine [USAN:BAN:INN];2-Chloro-2-deoxyadenosine;
- PSA 119.31000
- LogP 0.28380
Synthetic route

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 60℃; for 11h; | 100% |
| With ammonia In methanol at 60℃; | 59% |
-
-
1448245-68-8
2-chloro-N6,N6-dibenzoyl-3′,5′-O-dibenzoyl-2′-deoxyadenosine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 0 - 20℃; for 12h; | 95% |
-
-
500225-62-7
9-(3,5-di-O-benzoyl-2-deoxy-β-D-erythro-pentofuranosyl)-2,6-dichloropurine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol; dichloromethane at 80℃; for 7h; | 94% |
-
-
146196-13-6
6-amino-2-chloro-9-(2'deoxy-3',5'-di-O-acetyl-β-D-erythro-pentofuranosyl)-9H-purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 2h; pH=8 - 9; | 93% |
| With ammonia In methanol at 0 - 20℃; | 0.62 g |
-
-
908006-51-9
2-chloro-6-amino-9-[3,5-di-O-(4-chlorobenzoyl)-2-deoxy-β-D-ribofuranosyl]-purine

-
-
1266098-30-9
2-chloro-6-trimethylsilylamino-9-[3,5-di-O-(4-chlorobenzoyl)-2-deoxy-β-D-ribofuranosyl]-purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20 - 30℃; for 6h; | 90% |

| Conditions | Yield |
|---|---|
| With magnesium hydroxide; purine nucleoside phosphorylase In water at 45℃; | 88% |
-
-
24638-92-4
9-(3,5-di-O-acetyl-2-deoxy-β-D-erythro-pentofuranosyl)-2,6-dichloropurine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol; dichloromethane at 80℃; for 7h; | 87% |
-
-
38925-80-3
2,6-Dichloro-9-(2-deoxy-3,5-di-O-p-toluoyl-β-D-erythro-pentofuranosyl)-purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 110℃; for 5h; Sealed tube; | 86% |
| With ammonia In methanol at 100℃; for 5h; | 71% |
-
-
914084-44-9
2-chloro-9-[2-deoxy-3,5-di-O-(p-toluoyl)-β-D-erythro-pentofuranosyl]-6-(2-pentylimidazol-1-yl)purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 60℃; for 13h; | 85% |
-
-
914084-41-6
2-chloro-9-[2-deoxy-3,5-di-O-(p-toluoyl)-β-D-erythro-pentofuranosyl]-6-(2-propylimidazol-1-yl)purine

-
-
67-56-1
methanol

-
A
-
146196-07-8
2-chloro-9-(2'deoxy-β-D-erythro-pentofuranosyl)-6-methoxy-9H-purine

-
B
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia at 40℃; for 15h; | A 85% B n/a |

-
-
500225-59-2
9-(3,5-di-O-benzoyl-2-deoxy-β-D-erythro-pentofuranosyl)-2-chloro-6-O-(2,4,6-triisopropylbenzenesulfonyl)purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol; dichloromethane at 80℃; for 7h; | 83% |

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 50℃; for 10h; | 82% |

| Conditions | Yield |
|---|---|
| With 1A cells; E. coli BMT 4D In phosphate buffer at 65℃; for 4h; pH=7.5; | 81% |
| With purine nucleoside 2’-deoxyribosyltransferase from Trypanosoma brucei immobilized on glutaraldehyde-activated MagReSynAmine microspheres In aq. phosphate buffer at 50℃; for 0.333333h; pH=6; Enzymatic reaction; |
-
-
37390-66-2
2,6-dichloro 2’-deoxyriboside

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In acetonitrile at 20℃; for 24h; | 79% |
-
-
1384553-30-3
2,6-di-chloro-9-(3',5'-di-O-p-methoxybenzoyl-2'-deoxy-D-ribofuranosyl)purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With methanol; ammonia In dichloromethane at 80℃; | 79% |

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 10 - 50℃; for 1.33333h; Heating / reflux; | 72% |
| With sodium methylate In methanol at 20 - 50℃; for 0.333333h; | 72% |

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With potassium fluoride In methanol at 80℃; for 24h; | 72% |
-
-
98693-55-1, 102783-28-8
α-D-2′-deoxyribofuranose-1-O-phosphate bis(cyclohexylammonium) salt

-
-
1839-18-5
2-chloroadenine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With purine nucleoside phosphorylase; calcium hydroxide at 45℃; for 48h; pH=9.3; Enzymatic reaction; | 72% |
-
-
914084-44-9
2-chloro-9-[2-deoxy-3,5-di-O-(p-toluoyl)-β-D-erythro-pentofuranosyl]-6-(2-pentylimidazol-1-yl)purine

-
A
-
5542-92-7
6-amino-2-chloro-9-(2-deoxy-α-D-erythro-pentofuranosyl)purine

-
B
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 80℃; for 13h; | A n/a B 70% |

| Conditions | Yield |
|---|---|
| With potassium dihydrogenphosphate; Escherichia coli purine nucleoside phosphorylase In aq. buffer at 20℃; for 144h; pH=7.5; Enzymatic reaction; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: thymidine With magnesium(II) chloride hexahydrate; E. coli thymidine phosphorylase In aq. phosphate buffer at 40℃; for 120h; pH=7.20; QAE Sephadex-nPi; Stage #2: 2-chloroadenine With recombinant E. coli purine nucleoside phosphorylase In aq. phosphate buffer at 40℃; for 96h; pH=8.0; QAE Sephadex-nPi; | 59% |
| With immobilized recombinant Geobacillus stearothermophilus BKM-2194 purine nucleoside phosphorylase II/pyrimidine nucleoside phosphorylase In dimethyl sulfoxide at 75℃; for 4h; pH=7.5; aq. phosphate buffer; Enzymatic reaction; | 86 %Chromat. |
| With potassium phosphate; Geobacillus thermoglucosidasius purine nucleoside phosphorylase; Thermus thermophilus pyrimidine nucleoside phosphorylase In water at 70℃; for 1h; pH=7; Enzymatic reaction; |
-
-
500225-60-5
9-(3,5-di-O-benzoyl-2-deoxy-β-D-erythro-pentofuranosyl)-2-chloro-6-O-(6-methylbenzenesulfonyl)purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol; dichloromethane at 80℃; for 7h; | 43% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide 1.) EtOH, DMSO, alginate gel-entrapped cells of auxotropHic thymine-dependent strain of E. coli, acetate buffer pH 5.8, 37 deg C, 16 h, 2.) room temperature, overnight; Yield given. Multistep reaction; |
-
-
4330-21-6
2-deoxy-3,5-di-O-p-toluoyl-α-D-erythro-pentofuranosyl chloride

-
-
5451-40-1
2,6-dichloro-7H-purine

-
A
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia; sodium hydride Yield given. Multistep reaction. Yields of byproduct given; | |
| Stage #1: 2,6-dichloro-7H-purine With sodium hydride In acetonitrile at 20℃; for 1.5h; Inert atmosphere; Stage #2: 2-deoxy-3,5-di-O-p-toluoyl-α-D-erythro-pentofuranosyl chloride In acetonitrile for 24.5h; Inert atmosphere; Stage #3: With ammonia In methanol at 100℃; for 6h; | A 0.4 g B 0.1 g |

-
-
500225-58-1
9-(3,5-di-O-acetyl-2-deoxy-β-D-erythro-pentofuranosyl)-2-chloro-6-O-(2,4,6-triisopropylbenzenesulfonyl)purine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol; dichloromethane at 80℃; for 7h; |
-
-
136850-20-9
2-Chloro-9-((2R,3aS,9aR)-5,5,7,7-tetraisopropyl-tetrahydro-1,4,6,8-tetraoxa-5,7-disila-cyclopentacycloocten-2-yl)-9H-purin-6-ylamine

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| With ammonium fluoride In methanol Heating; |

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / purine nucleoside phosphorylase / H2O / 45 °C 2: 79 percent / aq. NH3 / acetonitrile / 24 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / NH3 / methanol / 24 h / 160 °C 2: 88 percent / purine nucleoside phosphorylase; Mg(OH)2 / H2O / 45 °C View Scheme | |
| Multi-step reaction with 6 steps 1: SnCl4 / acetonitrile / 20 °C 2: NH3 / methanol / 20 °C 3: pyridine / 20 °C 4: DMAP / acetonitrile / 20 °C 5: (Me3Si)3SiH; AIBN / dioxane / Heating 6: NH4F / methanol / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 67 percent / NH3, MeOH / 12 h / 100 °C 2: 75 percent / dimethylformamide / Ambient temperature 3: 2.) aq. NH3 / 1.) EtOH, DMSO, alginate gel-entrapped cells of auxotropHic thymine-dependent strain of E. coli, acetate buffer pH 5.8, 37 deg C, 16 h, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 2 steps 1: 59 percent / 1.) NaH / acetonitrile / 1.) r.t., 30 min; 2.) 15 h 2: 71 percent / ammonia / methanol / 5 h / 100 °C View Scheme |

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / NH3 / methanol / 24 h / 20 °C 2: 91 percent / purine nucleoside phosphorylase / H2O / 45 °C 3: 79 percent / aq. NH3 / acetonitrile / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 92 percent / NH3 / methanol / 24 h / 20 °C 2: 88 percent / purine nucleoside phosphorylase; Mg(OH)2 / H2O / 45 °C View Scheme |
-
-
108-24-7
acetic anhydride

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
146196-13-6
6-amino-2-chloro-9-(2'deoxy-3',5'-di-O-acetyl-β-D-erythro-pentofuranosyl)-9H-purine

| Conditions | Yield |
|---|---|
| With pyridine for 3h; | 92% |
| With pyridine for 2h; Ambient temperature; | 81% |
-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
404839-80-1
2-azido-9-(2-deoxy-β-D-erythro-pentofuranosyl)adenine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-2'-deoxyadenosine With hydrazine hydrate at 20℃; for 16h; Stage #2: With acetic acid; sodium nitrite In water for 1h; cooling; | 91% |
| Multi-step reaction with 2 steps 1: H2NNH2*H2O / 20 °C 2: NaNO2; acetic acid / H2O / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| In methanol at 130℃; for 24h; | 90% |
-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
156834-09-2
2-chloro-9-<2-deoxy-5-O-<(1,1-dimethylethyl)diphenylsilyl>-β-D-erythro-pentofuranosyl>-9H-purin-6-amine

| Conditions | Yield |
|---|---|
| With pyridine for 14h; | 83% |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
136850-20-9
2-Chloro-9-((2R,3aS,9aR)-5,5,7,7-tetraisopropyl-tetrahydro-1,4,6,8-tetraoxa-5,7-disila-cyclopentacycloocten-2-yl)-9H-purin-6-ylamine

| Conditions | Yield |
|---|---|
| With pyridine at 5 - 20℃; for 1.5h; | 83% |
-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
1188-33-6
N,N-dimethylformamide diethyl diacetal

-
-
146196-18-1
2-chloro-9-(2'-deoxy-β-D-erythro-pentofuranosyl)-6-<<(dimethylamino)methylidene>amino>-9H-purine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; | 82% |
-
-
107-18-6
allyl alcohol

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
916755-74-3
2-allyloxy-6-amino-9-[2-deoxy-β-D-erythro-pentofuranosyl]-9H-purine

| Conditions | Yield |
|---|---|
| With sodium allyloxide at 60℃; for 2h; | 79% |
| Stage #1: allyl alcohol With sodium In paraffin oil at 0℃; for 1h; Inert atmosphere; Stage #2: 2-chloro-2'-deoxyadenosine In paraffin oil at 88℃; for 12h; | 62% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
4291-63-8
2-chloro-2'-deoxyadenosine

-
-
128126-38-5
5'-O-tert-butyldimethylsilylcladribine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 20h; Inert atmosphere; | 75% |
| With 1H-imidazole In pyridine at 20℃; |

| Conditions | Yield |
|---|---|
| In methanol at 130℃; for 24h; | 75% |

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine at 0 - 20℃; for 24h; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: benzyl alcohol With sodium In paraffin oil at 80℃; for 0.75h; Inert atmosphere; Stage #2: 2-chloro-2'-deoxyadenosine With silicon In acetonitrile; paraffin oil at 85℃; for 12h; | 71% |
Cladribine Specification
1. Introduction of Cladribine
Cladribine (CAS NO.4291-63-8) is a white crystalline solid. Its IUPAC Name is (2R,3S,5R)-5-(6-amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol. Its water solubility is 6.35e+00 g/l. The Classification Code is Antineoplastic; Antineoplastic agents; Drug / Therapeutic Agent; Human Data; Immunologic Factors; Immunosuppressive agents; Mutation data; Reproductive Effect. Cladribine belongs to Bases & Related Reagents;Nucleotides;Pharmaceuticals;API. Besides, it should be stored in Freezer.
2. Properties of Cladribine
Physical properties about Cladribine are:
(1)Index of Refraction: 1.87; (2)Molar Refractivity: 63.7 cm3; (3)Molar Volume: 140.1 cm3; (4)Polarizability: 25.25×10-24cm3; (5)Surface Tension: 93.5 dyne/cm; (6)Density: 2.03 g/cm3; (7)Flash Point: 285 °C; (8)Enthalpy of Vaporization: 87.03 kJ/mol; (9)Melting Point: 181-185 °C(lit.); (10)Boiling Point: 547.6 °C at 760 mmHg; (11)Vapour Pressure: 7.95E-13 mmHg at 25°C.
3. Structure Descriptors of Cladribine
(1)Canonical SMILES: C1C(C(OC1N2C=NC3=C2N=C(N=C3N)Cl)CO)O
(2)Isomeric SMILES: C1[C@@H]([C@H](O[C@H]1N2C=NC3=C2N=C(N=C3N)Cl)CO)O
(3)InChI: InChI=1S/C10H12ClN5O3/c11-10-14-8(12)7-9(15-10)16(3-13-7)6-1-4(18)5(2-17)19-6/h3-6,17-18H,1-2H2,(H2,12,14,15)/t4-,5+,6+/m0/s1
(4)InChIKey: PTOAARAWEBMLNO-KVQBGUIXSA-N
4. Toxicity of Cladribine
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | TDLo | intravenous | 700ug/kg/7D-C (0.7mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | American Journal of Hematology. Vol. 53, Pg. 209, 1996. |
| mouse | LD50 | intraperitoneal | 150mg/kg (150mg/kg) | Leukemia and Lymphoma. Vol. 5, Pg. 1, 1991. |
5. Safety information of Cladribine
Hazard Codes:
 T
T Risk Statements: 36/37/38-23/24/25
R36/37/38:Irritating to eyes, respiratory system and skin.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-37/39-45-36-22
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39:Wear suitable gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36:Wear suitable protective clothing.
S22:Do not breathe dust.
WGK Germany: 3
RTECS: AU7357560
6. Uses of Cladribine
Cladribine is a substituted purine nucleoside with antileukemic activity. Cladribine is a drug used to treat hairy cell leukemia. As a purine analog, it is a synthetic anti-cancer agent that also suppresses the immune system. Chemically, it mimics the nucleoside adenosine and thus inhibits the enzyme adenosine deaminase, which interferes with the cell's ability to process DNA.
7. Production of Cladribine
Cladribine can be got from 2-Deoxy-D-ribose. The detail is as follows:
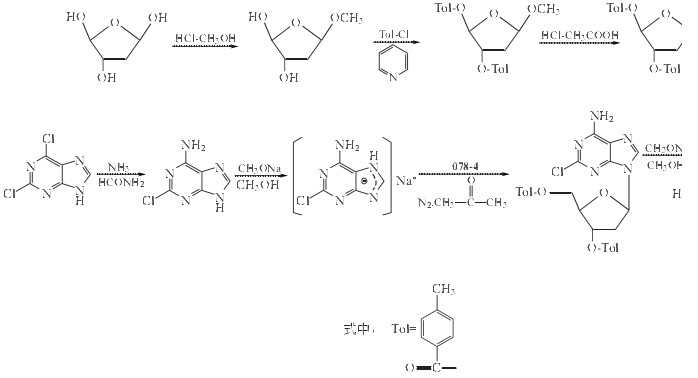
Related Products
- Cladribine
- 42916-73-4
- 42918-86-5
- 42918-89-8
- 4291-99-0
- 4292-10-8
- 4292-12-0
- 4292-19-7
- 42923-76-2
- 42923-77-3
- 42923-79-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View