-
Name
Homovanillyl alcohol
- EINECS 219-175-1
- CAS No. 2380-78-1
- Article Data23
- CAS DataBase
- Density 1.182 g/cm3
- Solubility Soluble in water
- Melting Point 40-42 °C(lit.)
- Formula C9H12O3
- Boiling Point 316.8 °C at 760 mmHg
- Molecular Weight 168.192
- Flash Point 145.4 °C
- Transport Information
- Appearance yellow to light brown crystalline chunks
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Phenethylalcohol, 4-hydroxy-3-methoxy- (6CI,7CI,8CI);2-(3-Methoxy-4-hydroxyphenyl)ethanol;2-(4-Hydroxy-3-methoxyphenyl)ethanol;3-Methoxy-4-hydroxyphenethanol;3-Methoxy-4-hydroxyphenethyl alcohol;4-(2-Hydroxyethyl)-2-methoxyphenol;4-(2-Hydroxyethyl)guaiacol;4-Hydroxy-3-methoxyphenethyl alcohol;Ba 2772;Homovanillic alcohol;Homovanillyl alcohol;Vanillylmethanol;
- PSA 49.69000
- LogP 0.93560
Synthetic route

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In ethanol for 50h; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-allylguaiacol With ozone In ethanol at -55℃; Stage #2: With sodium tetrahydroborate In ethyl acetate at 20℃; | 98% |
| Multi-step reaction with 2 steps 1: ozone / sodium hydrogencarbonate / dichloromethane; methanol / 2 h / -78 °C 2: sodium tetrahydroborate; methanol / dichloromethane / 2 h / -78 - 20 °C / Inert atmosphere View Scheme |
-
-
124-41-4
sodium methylate

-
-
196081-78-4
3-bromo-4-hydroxyphenylethyl alcohol

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With copper(I) bromide In N,N-dimethyl-formamide at 120℃; for 1h; | 97.3% |
| Stage #1: sodium methylate With copper(I) bromide In methanol; N,N-dimethyl-formamide Stage #2: 3-bromo-4-hydroxyphenylethyl alcohol In methanol; N,N-dimethyl-formamide at 100℃; Temperature; | 92% |
| With methanol; copper(I) bromide In N,N-dimethyl-formamide at 90℃; for 3h; | 87.6% |
| With copper(I) bromide In water; N,N-dimethyl-formamide | |
| copper(I) bromide In methanol; N,N-dimethyl-formamide at 120℃; |

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In ethanol for 50h; Microwave irradiation; | 94% |
-
-
1044264-42-7
C21H40O3Si2

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With methanol; trimethylsilyl bromide at 20℃; for 5h; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With β-D-glucose; oxygen In aq. phosphate buffer at 37℃; for 18h; pH=7.0; Enzymatic reaction; | 91.9% |
| Multi-step reaction with 4 steps 1: Bacillus licheniformis strain CGMCC 7172 phenolic acid decarboxylase / 6 h / Enzymatic reaction 2: oxygen; β-D-glucose; styrene monooxygenase (AF031161.1) from Pseudomonas sp. VLB120 / Enzymatic reaction 3: styrene oxide isomerase (KF540254.1) from Rhodococcus opacus 1CP / Enzymatic reaction 4: alcohol dehydrogenase (NC_001145.3) from Saccharomyces cerevisiae S288C View Scheme |
-
-
64881-05-6
1-<4-(benzyloxy)-3-methoxyphenyl>ethan-2-ol

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 750.06 Torr; for 1h; | 91% |
-
-
60563-13-5
ethyl homovanillate

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 3h; Inert atmosphere; Reflux; | 89% |
-
-
67-56-1
methanol

-
-
94492-22-5
cistanoside C

-
A
-
2380-78-1
homovanillyl alcohol

-
B
-
3843-74-1, 67667-67-8
Methyl caffeate

| Conditions | Yield |
|---|---|
| With acetyl chloride for 0.5h; Heating; |

-
-
67-56-1
methanol

-
-
94492-21-4
cistanoside D

-
A
-
2380-78-1
homovanillyl alcohol

-
B
-
2309-07-1
Methyl ferulate

| Conditions | Yield |
|---|---|
| With acetyl chloride for 0.5h; Heating; |

-
-
93236-41-0
cistanoside

-
-
75-36-5
acetyl chloride

-
A
-
97966-29-5
methyl (E)-3-(3-hydroxy-4-methoxyphenyl)-2-propenoate

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| In methanol for 0.5h; Heating; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| In methanol for 0.5h; Heating; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| (i) nBuLi, Et2O, (ii) /BRN= 102378/, Et2O, benzene; Multistep reaction; |
-
-
63057-72-7
2-benzyloxy-5-bromoanisole

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) Mg, 1,2-dibromoethane, 2.) 1,5-cyclooctadiene copper(I) chloride / 1.) THF, reflux, 5 h, 2.) THF, 0 deg C - 14 deg C, 16 h 2: 91 percent / H2 / 5percent Pd/C / methanol / 1 h / 750.06 Torr View Scheme |

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In ethanol for 50h; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-allylguaiacol With ozone In ethanol at -55℃; Stage #2: With sodium tetrahydroborate In ethyl acetate at 20℃; | 98% |
| Multi-step reaction with 2 steps 1: ozone / sodium hydrogencarbonate / dichloromethane; methanol / 2 h / -78 °C 2: sodium tetrahydroborate; methanol / dichloromethane / 2 h / -78 - 20 °C / Inert atmosphere View Scheme |
-
-
124-41-4
sodium methylate

-
-
196081-78-4
3-bromo-4-hydroxyphenylethyl alcohol

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With copper(I) bromide In N,N-dimethyl-formamide at 120℃; for 1h; | 97.3% |
| Stage #1: sodium methylate With copper(I) bromide In methanol; N,N-dimethyl-formamide Stage #2: 3-bromo-4-hydroxyphenylethyl alcohol In methanol; N,N-dimethyl-formamide at 100℃; Temperature; | 92% |
| With methanol; copper(I) bromide In N,N-dimethyl-formamide at 90℃; for 3h; | 87.6% |
| With copper(I) bromide In water; N,N-dimethyl-formamide | |
| copper(I) bromide In methanol; N,N-dimethyl-formamide at 120℃; |

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In ethanol for 50h; Microwave irradiation; | 94% |
-
-
1044264-42-7
C21H40O3Si2

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With methanol; trimethylsilyl bromide at 20℃; for 5h; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With β-D-glucose; oxygen In aq. phosphate buffer at 37℃; for 18h; pH=7.0; Enzymatic reaction; | 91.9% |
| Multi-step reaction with 4 steps 1: Bacillus licheniformis strain CGMCC 7172 phenolic acid decarboxylase / 6 h / Enzymatic reaction 2: oxygen; β-D-glucose; styrene monooxygenase (AF031161.1) from Pseudomonas sp. VLB120 / Enzymatic reaction 3: styrene oxide isomerase (KF540254.1) from Rhodococcus opacus 1CP / Enzymatic reaction 4: alcohol dehydrogenase (NC_001145.3) from Saccharomyces cerevisiae S288C View Scheme |
-
-
64881-05-6
1-<4-(benzyloxy)-3-methoxyphenyl>ethan-2-ol

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 750.06 Torr; for 1h; | 91% |
-
-
60563-13-5
ethyl homovanillate

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 3h; Inert atmosphere; Reflux; | 89% |
-
-
67-56-1
methanol

-
-
94492-22-5
cistanoside C

-
A
-
2380-78-1
homovanillyl alcohol

-
B
-
3843-74-1, 67667-67-8
Methyl caffeate

| Conditions | Yield |
|---|---|
| With acetyl chloride for 0.5h; Heating; |

-
-
67-56-1
methanol

-
-
94492-21-4
cistanoside D

-
A
-
2380-78-1
homovanillyl alcohol

-
B
-
2309-07-1
Methyl ferulate

| Conditions | Yield |
|---|---|
| With acetyl chloride for 0.5h; Heating; |

-
-
93236-41-0
cistanoside

-
-
75-36-5
acetyl chloride

-
A
-
97966-29-5
methyl (E)-3-(3-hydroxy-4-methoxyphenyl)-2-propenoate

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| In methanol for 0.5h; Heating; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| In methanol for 0.5h; Heating; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| (i) nBuLi, Et2O, (ii) /BRN= 102378/, Et2O, benzene; Multistep reaction; |
-
-
63057-72-7
2-benzyloxy-5-bromoanisole

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) Mg, 1,2-dibromoethane, 2.) 1,5-cyclooctadiene copper(I) chloride / 1.) THF, reflux, 5 h, 2.) THF, 0 deg C - 14 deg C, 16 h 2: 91 percent / H2 / 5percent Pd/C / methanol / 1 h / 750.06 Torr View Scheme |
-
-
1286729-19-8
1-tert-butoxy-4-(2-tert-butoxyethyl)-2-methoxybenzene

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In methanol at 100℃; for 0.75h; Microwave irradiation; |

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate In dichloromethane at -78 - 20℃; for 2h; Inert atmosphere; | |
| With alcohol dehydrogenase (NC_001145.3) from Saccharomyces cerevisiae S288C |

-
A
-
645-56-7
4-n-Propylphenol

-
B
-
93-51-6
2-Methoxy-4-methylphenol

-
C
-
2785-87-7
2-methoxy-4-n-propylphenol

-
D
-
97-54-1
2-methoxy-4-propenylphenol

-
E
-
2785-89-9
4-Ethylguaiacol

-
F
-
2380-78-1
homovanillyl alcohol

-
G
-
2525-02-2
4-propylbenzene-1,2-diol

-
H
-
120-80-9
benzene-1,2-diol

-
I
-
90-05-1
2-methoxy-phenol

-
J
-
452-86-8
4-methyl-1,2-dihydroxybenzene

| Conditions | Yield |
|---|---|
| With 5% palladium on Al2O3; hydrogen In water at 25 - 310℃; under 37503.8 Torr; for 1h; Autoclave; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium bromide; oxone / acetone; water / 20 °C 2.1: copper(I) bromide / N,N-dimethyl-formamide; methanol 2.2: 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium bromide; oxone||potassium monopersulfate triple salt / acetone; water / 15 h / 0 °C 2: copper(I) bromide; methanol / N,N-dimethyl-formamide / 3 h / 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: N-Bromosuccinimide / methanol / 3 h / 20 °C 2.1: copper(I) bromide; sodium methylate / methanol / 15 h / 100 °C 2.2: 12 h / 20 °C View Scheme |
-
-
25379-88-8
2-(3,4-dihydroxyphenyl)acetic acid methyl ester

-
A
-
50602-41-0
2-(3-hydroxy-4-methoxyphenyl)ethanol

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: toluene-4-sulfonic acid / dichloromethane / 8 h / Inert atmosphere; Darkness; Reflux 2: lithium aluminium tetrahydride / diethyl ether / 3 h / 0 °C / Inert atmosphere 3: Amberlyst 15 / methanol / 8 h / Reflux 4: methyltransferase I from Desulfitobacterium hafniense / methanol / 24 h / 30 °C / pH 6.5 / Inert atmosphere; Glovebox; Enzymatic reaction View Scheme |
-
-
10597-60-1
hydroxytyrosol

-
-
90-05-1
2-methoxy-phenol

-
A
-
50602-41-0
2-(3-hydroxy-4-methoxyphenyl)ethanol

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With methyltransferase I from Desulfitobacterium hafniense In methanol at 30℃; for 24h; pH=6.5; Solvent; Inert atmosphere; Glovebox; Enzymatic reaction; regioselective reaction; |

-
-
38515-62-7
2-(2,2-dimethylbenzo[1,3]dioxol-5-yl)acetic acid methyl ester

-
A
-
50602-41-0
2-(3-hydroxy-4-methoxyphenyl)ethanol

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: lithium aluminium tetrahydride / diethyl ether / 3 h / 0 °C / Inert atmosphere 2: Amberlyst 15 / methanol / 8 h / Reflux 3: methyltransferase I from Desulfitobacterium hafniense / methanol / 24 h / 30 °C / pH 6.5 / Inert atmosphere; Glovebox; Enzymatic reaction View Scheme |
-
-
119054-91-0
2-(2,2-dimethylbenzo[d][1,3]dioxol-5-yl)ethan-1-ol

-
A
-
50602-41-0
2-(3-hydroxy-4-methoxyphenyl)ethanol

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Amberlyst 15 / methanol / 8 h / Reflux 2: methyltransferase I from Desulfitobacterium hafniense / methanol / 24 h / 30 °C / pH 6.5 / Inert atmosphere; Glovebox; Enzymatic reaction View Scheme |
-
-
102-32-9
3,4-dihydroxyphenylacetate

-
A
-
50602-41-0
2-(3-hydroxy-4-methoxyphenyl)ethanol

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sulfuric acid / 2 h / Inert atmosphere; Darkness; Reflux 2: toluene-4-sulfonic acid / dichloromethane / 8 h / Inert atmosphere; Darkness; Reflux 3: lithium aluminium tetrahydride / diethyl ether / 3 h / 0 °C / Inert atmosphere 4: Amberlyst 15 / methanol / 8 h / Reflux 5: methyltransferase I from Desulfitobacterium hafniense / methanol / 24 h / 30 °C / pH 6.5 / Inert atmosphere; Glovebox; Enzymatic reaction View Scheme |

-
A
-
2033-89-8
3,4-dimethoxyphenol

-
B
-
97-53-0
4-allylguaiacol

-
C
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
D
-
93-51-6
2-Methoxy-4-methylphenol

-
E
-
2785-87-7
2-methoxy-4-n-propylphenol

-
F
-
934-00-9
3-methocycatechol

-
G
-
634-36-6
1,2,3-trimethoxybenzene

-
H
-
480-66-0
2,4,6-trihydroxyacetophenone

-
I
-
6627-88-9
4-allyl-2,6-dimethoxyphenol

-
J
-
2478-38-8
1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone

-
K
-
7786-61-0
3-methoxy-4-hydroxystyrene

-
L
-
2785-89-9
4-Ethylguaiacol

-
M
-
2503-46-0
1-(4-hydroxy-3-methoxyphenyl)2-propanone

-
N
-
1509-06-4
1-(2,4,6-trihydroxy-3-methylphenyl)butan-1-one

-
O
-
20481-17-8
3-hydroxy-5-tert-butyl-catechol

-
P
-
2380-78-1
homovanillyl alcohol

-
Q
-
120-80-9
benzene-1,2-diol

-
R
-
90-05-1
2-methoxy-phenol

-
S
-
452-86-8
4-methyl-1,2-dihydroxybenzene

-
T
-
134-96-3
syringic aldehyde

-
U
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| at 500℃; for 0.00555556h; Pyrolysis; |


-
A
-
97-53-0
4-allylguaiacol

-
B
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
C
-
93-51-6
2-Methoxy-4-methylphenol

-
D
-
2785-87-7
2-methoxy-4-n-propylphenol

-
E
-
526-75-0
2,3-Dimethylphenol

-
F
-
934-00-9
3-methocycatechol

-
G
-
634-36-6
1,2,3-trimethoxybenzene

-
H
-
7507-89-3
2,6-dihydroxy-4-methoxy-acetophenone

-
I
-
6627-88-9
4-allyl-2,6-dimethoxyphenol

-
J
-
2478-38-8
1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone

-
K
-
7786-61-0
3-methoxy-4-hydroxystyrene

-
L
-
2785-89-9
4-Ethylguaiacol

-
M
-
2503-46-0
1-(4-hydroxy-3-methoxyphenyl)2-propanone

-
N
-
2380-78-1
homovanillyl alcohol

-
O
-
120-80-9
benzene-1,2-diol

-
P
-
90-05-1
2-methoxy-phenol

-
Q
-
134-96-3
syringic aldehyde

-
R
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| at 500℃; for 0.00555556h; Pyrolysis; |

-
-
501-94-0
p-hydroxyphenethyl alcohol

-
A
-
953422-35-0
2-(4'-hydroxy-3'-methoxyphenyl)ethyl methyl carbonate

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / methanol / 3 h / 20 °C 2: copper(I) bromide; sodium methylate / methanol / 15 h / 100 °C View Scheme |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
196081-78-4
3-bromo-4-hydroxyphenylethyl alcohol

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: carbonic acid dimethyl ester; 3-bromo-4-hydroxyphenylethyl alcohol With sodium methylate; copper(I) bromide In methanol at 100℃; for 15h; Stage #2: With potassium carbonate In methanol at 20℃; for 12h; |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
196081-78-4
3-bromo-4-hydroxyphenylethyl alcohol

-
A
-
953422-35-0
2-(4'-hydroxy-3'-methoxyphenyl)ethyl methyl carbonate

-
B
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With sodium methylate; copper(I) bromide In methanol at 100℃; for 15h; |

-
-
61914-52-1
1-(3,4-dimethoxyphenyl)-5-hydroxydecan-3-one

-
A
-
2380-78-1
homovanillyl alcohol

-
E
-
7417-21-2
2-(3,4-dimethoxyphenyl)ethyl alcohol

| Conditions | Yield |
|---|---|
| With Colletotrichum gloeosporioides In water; dimethyl sulfoxide at 30℃; for 120h; Microbiological reaction; |

-
-
2380-78-1
homovanillyl alcohol

-
-
108-24-7
acetic anhydride

-
-
32022-28-9
4-(2-acetoxyethyl)-2-methoxy-1-acetoxy-benzene

| Conditions | Yield |
|---|---|
| In neat (no solvent) Molecular sieve; Microwave irradiation; Green chemistry; | 100% |
| With acetic acid at 50℃; | 95% |
| With acetic acid at 50℃; | 95.3% |
| With pyridine | 95% |
| With acetic acid at 50℃; for 12h; | 67% |
-
-
2380-78-1
homovanillyl alcohol

-
-
616-38-6
carbonic acid dimethyl ester

-
-
953422-35-0
2-(4'-hydroxy-3'-methoxyphenyl)ethyl methyl carbonate

| Conditions | Yield |
|---|---|
| With sulfuric acid for 7h; Reflux; | 100% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene at 90℃; for 7.5h; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 3h; | 100% |
| With 1H-imidazole |

| Conditions | Yield |
|---|---|
| In carbonic acid dimethyl ester at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase In various solvent(s) at 40℃; for 1.5h; | 98.1% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase In various solvent(s) at 40℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase In various solvent(s) at 40℃; for 1.5h; | 97.5% |
-
-
2380-78-1
homovanillyl alcohol

-
-
10597-60-1
hydroxytyrosol

| Conditions | Yield |
|---|---|
| With 3-mercaptopropionic acid ethyl ester In aq. buffer at 30℃; for 24h; pH=6.5; Inert atmosphere; Glovebox; Enzymatic reaction; | 97% |
| Stage #1: homovanillyl alcohol With aluminum (III) chloride In ethanethiol at 0 - 20℃; for 42h; Stage #2: With hydrogenchloride; water Cooling with ice; | 94.7% |
| With sodium periodate In water; ethyl acetate Concentration; Reagent/catalyst; Temperature; Solvent; Time; | 78% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase In various solvent(s) at 40℃; for 1h; | 96.8% |
| With Candida antarctica lipase In tert-butyl methyl ether at 40℃; Enzymatic reaction; | 96.5% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 7h; Reflux; | 95% |
-
-
5963-14-4
8-methylnonanoic acid

-
-
2380-78-1
homovanillyl alcohol

-
-
951221-74-2
8-methylnonanoic acid 2-(4-hydroxy-3-methoxyphenyl)ethyl ester

| Conditions | Yield |
|---|---|
| novozyme 435 at 50℃; for 16h; Enzymatic reaction; Neat (no solvent); | 93.2% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide for 0.5h; | 93% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2380-78-1
homovanillyl alcohol

| Conditions | Yield |
|---|---|
| With mesoporous silica MCM-41 supported erbium(III) at 40℃; for 9h; Neat (no solvent); ultrasound irradiation; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase In various solvent(s) at 40℃; for 1.5h; | 90.9% |

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; calcium chloride; diothiothreitol; benzenesulfonamide; uridine 5'-diphosphoglucuronyl transferases (porcine liver) In water; dimethyl sulfoxide at 35℃; for 6h; pH=8.0; Enzymatic reaction; | A 88% B 6.1% |

Homovanillyl alcohol Specification
The CAS register number of Homovanillyl alcohol is 2380-78-1. It also can be called as 4-Hydroxy-3-methoxyphenethyl alcohol and the IUPAC name about this chemical is 4-(2-hydroxyethyl)-2-methoxyphenol. It belongs to the following product categories, such as Benzhydrols, Benzyl & Special Alcohols, Alcohols, Anisoles, Alkyloxy Compounds & Phenylacetates, Fluorine Compounds, Bromine Compounds, Iodine Compounds and so on. This chemical is a metabolite of serotonin and norepinephrine.
Physical properties about Homovanillyl alcohol are: (1)ACD/LogP: 0.33; (2)ACD/LogD (pH 5.5): 0.33; (3)ACD/LogD (pH 7.4): 0.33; (4)ACD/BCF (pH 5.5): 1.04; (5)ACD/BCF (pH 7.4): 1.04; (6)ACD/KOC (pH 5.5): 35.9; (7)ACD/KOC (pH 7.4): 35.85; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 5; (11)Polar Surface Area: 27.69Å2; (12)Index of Refraction: 1.558; (13)Molar Refractivity: 45.89 cm3; (14)Molar Volume: 142.1 cm3; (15)Polarizability: 18.19x10-24cm3; (16)Surface Tension: 46.9 dyne/cm; (17)Enthalpy of Vaporization: 58.93 kJ/mol; (18)Boiling Point: 316.8 °C at 760 mmHg; (19)Vapour Pressure: 0.000169 mmHg at 25°C.
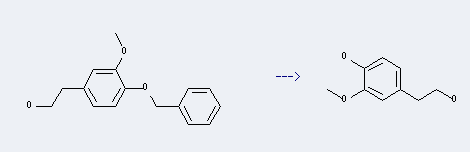
Preparation: this chemical can be prepared by 4-benzyloxy-3-methoxyphenethyl alcohol. This reaction will need reagent H2, catalyst 5percent Pd/C and solvent methanol. The reaction time is 1 hour(s) with reaction pressure of 750.06. The yield is about 91 %.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Oc1ccc(cc1OC)CCO
(2)InChI: InChI=1/C9H12O3/c1-12-9-6-7(4-5-10)2-3-8(9)11/h2-3,6,10-11H,4-5H2,1H3
(3)InChIKey: XHUBSJRBOQIZNI-UHFFFAOYAP
(4)Std. InChI: InChI=1S/C9H12O3/c1-12-9-6-7(4-5-10)2-3-8(9)11/h2-3,6,10-11H,4-5H2,1H3
(5)Std. InChIKey: XHUBSJRBOQIZNI-UHFFFAOYSA-N
Related Products
- Homovanillyl alcohol
- 23808-42-6
- 23808-43-7
- 23808-46-0
- 2380-84-9
- 2380-86-1
- 238088-70-5
- 2380-91-8
- 2380-94-1
- 238098-26-5
- 2381-07-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View