-
Name
Imidazolyl-4-ethanol
- EINECS
- CAS No. 872-82-2
- Article Data14
- CAS DataBase
- Density 1.228 g/cm3
- Solubility
- Melting Point 82 °C
- Formula C5H8 N2 O
- Boiling Point 359.8ºC at 760 mmHg
- Molecular Weight 112.131
- Flash Point 171.4ºC
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1H-Imidazole-4-ethanol(9CI);Imidazole-4(or 5)-ethanol (6CI,7CI);Imidazole-4-ethanol (8CI);2-(1H-Imidazol-4-yl)ethanol;4-(2-Hydroxyethyl)imidazole;4-(b-Hydroxyethyl)imidazole;Histaminol;
- PSA 48.91000
- LogP -0.05550
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(II) sulfate | |
| With ammonium hydroxide; copper(I) sulfate In water at 80℃; for 2h; | 31.6 g |
-
-
90773-75-4
4-(2-hydroxy-ethyl)-1,3-dihydro-imidazole-2-thione

-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| With nitric acid |

| Conditions | Yield |
|---|---|
| With ethanol; diamine oxidase from pea seedling; liver alcohol dehydrogenase; NAD; catalase at 37℃; for 48h; phosphate buffer (pH 7); | 34 mg |
| With hydrogenchloride; sodium nitrite In water at 40℃; for 2h; Reagent/catalyst; Temperature; |

-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| With barium nitrite; water |
-
-
50-00-0
formaldehyd

-
-
140-86-3
1,4-dihydroxy-2-butanone

-
-
7664-41-7
ammonia

-
-
7732-18-5
water

-
-
7758-99-8
copper(II) sulfate

-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| at 70 - 80℃; |

-
-
51718-80-0
methyl 2-(1H-imidazol-4-yl)acetate hydrochloride

-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 3h; Heating / reflux; |
-
-
30290-08-5
4-(2′-methoxyethyl)imidazole

-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 1.5h; Reflux; | 5.41 g |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(1H-imidazol-4-yl)ethanol; tert-butyl 2-chloro-4-iodobenzoate With potassium phosphate; copper(l) iodide; (R,R)-N,N'-dimethyl-1,2-diaminocyclohexane In dimethyl sulfoxide at 50℃; for 2h; Stage #2: With hydrogenchloride In 1,4-dioxane at 70℃; for 15h; | 96% |

| Conditions | Yield |
|---|---|
| In methanol for 1h; Heating; | 58.8% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 140℃; for 12h; | 58% |
| With caesium carbonate In N,N-dimethyl-formamide at 140℃; for 12h; | 58% |

| Conditions | Yield |
|---|---|
| In methanol; water for 1h; Heating; | 55.4% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 24h; Heating; | 48% |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
76-83-5
trityl chloride

-
-
127607-62-9
1-triphenylmethyl-4-(2-hydroxyethyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 40% |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
221164-86-9
ethyl 7-fluoro-3-ethoxy-6-nitroquinoxaline-2-carboxylate

-
-
221167-10-8
ethyl 3-ethoxy-7-[4-(2-hydroxyethyl)imidazol-1-yl]-6-nitroquinoxaline-2-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl acetamide at 120℃; for 15h; | 28% |
| With triethylamine In N,N-dimethyl acetamide at 120℃; for 15h; | 28% |
| With triethylamine |

| Conditions | Yield |
|---|---|
| In methanol for 1h; Heating; | 26.8% |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
103-71-9
phenyl isocyanate

-
-
74294-66-9
Phenyl-carbamic acid 2-(1H-imidazol-4-yl)-ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine at 80℃; for 0.5h; | 803 mg |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
74294-60-3
NO-bis-p-tolylsulphonylhistaminol

| Conditions | Yield |
|---|---|
| With pyridine at -23℃; for 2h; | 336 mg |

| Conditions | Yield |
|---|---|
| UV-Licht.Irradiation; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
211503-47-8
tert-butyl 4-(2-hydroxyethyl)imidazole-1-carboxylate

| Conditions | Yield |
|---|---|
| With TEA In methanol at 20℃; |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
908015-61-2
4-(2-methanesulfonyloxy-ethyl)-imidazole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TEA / methanol / 20 °C 2: TEA / CH2Cl2 / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 336 mg / pyridine / 2 h / -23 °C 2: 180 mg / lithium azide / dimethylformamide / 18 h / 21 °C 3: 90 mg / aq. hydrochloric acid, hydrogen / palladium-charcoal / ethanol / 8 h View Scheme |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
74294-62-5
4-(2-Azido-ethyl)-1-(toluene-4-sulfonyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 336 mg / pyridine / 2 h / -23 °C 2: 180 mg / lithium azide / dimethylformamide / 18 h / 21 °C View Scheme |

| Conditions | Yield |
|---|---|
| In aqueous HBr |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
930768-34-6
2-[1-(toluene-4-sulfonyl)-1H-imidazol-4-yl]-ethanol

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; dichloromethane for 0.333333h; |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
1571145-65-7
4-(2-((tert-butyldiphenylsilyl)oxy)ethyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 2h; | 1.6 g |
| With 1H-imidazole at 20℃; for 2h; | 1.6 g |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| With trans-N,N'-dimethyl-1,2-cyclohexyldiamine; potassium phosphate; copper(l) iodide In dimethyl sulfoxide at 20 - 50℃; for 2h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / N,N-dimethyl-formamide / 12 h / 20 °C 2: sodium hydride / N,N-dimethyl-formamide / 4 h / 70 °C View Scheme |
-
-
872-82-2
2-(1H-imidazol-4-yl)ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: caesium carbonate / N,N-dimethyl-formamide / 12 h / 140 °C 2.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0.08 h 2.2: 0.5 h / 20 °C 3.1: phosphorus tribromide / dichloromethane / 20 °C View Scheme |
Imidazolyl-4-ethanol Specification
The 1H-Imidazole-5-ethanol, with its cas register number 872-82-2, has its IUPAC name of 2-(1H-imidazol-5-yl)ethanol. This chemical is a kind of white or alomost white solid, and this chemical belongs to the product category which is pharmacetical.
The physical properties of this chemical could be summarized as: (1)ACD/BCF (pH 5.5): 1; (2)ACD/BCF (pH 7.4): 1; (3)ACD/KOC (pH 5.5): 1; (4)ACD/KOC (pH 7.4): 5.696; (5)#H bond acceptors: 3; (6)#H bond donors: 2; (7)#Freely Rotating Bonds: 3; (8)Polar Surface Area: 48.91; (9)Index of Refraction: 1.568; (10)Molar Refractivity: 29.861 cm3; (11)Molar Volume: 91.303 cm3; (12)Polarizability: 11.838 ×10-24 cm3; (13)Surface Tension: 60.23 dyne/cm; (14)Density: 1.228 g/cm3; (15)Flash Point: 171.38 °C; (16)Enthalpy of Vaporization: 63.879 kJ/mol; (17)Boiling Point: 359.766 °C at 760 mmHg.
Use of 1H-Imidazole-5-ethanol: 1H-Imidazole-5-ethanol could react with 1H-pyridine-2-thione to produce 2-[2-(1H-imidazol-4-yl)-ethylsulfanyl]-pyridine; dihydrobromide.
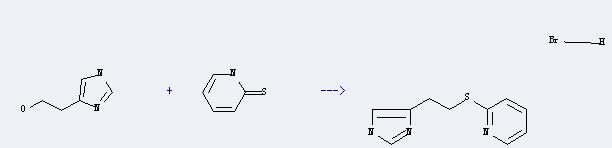
This reation could occur in the following condition: reagent: 47percent aq. HBr; reaction time: 24 hours; other condition: heating; field: 48%.
Additionally, you could convert the following datas information into the molecular structure:
(1)Canonical SMILES: C1=C(NC=N1)CCO
(2)InChI: InChI=1S/C5H8N2O/c8-2-1-5-3-6-4-7-5/h3-4,8H,1-2H2,(H,6,7)
(3)InChIKey: HEEACTTWORLLPM-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View