-
Name
Kojic acid
- EINECS 207-922-4
- CAS No. 501-30-4
- Article Data18
- CAS DataBase
- Density 1.542 g/cm3
- Solubility Soluble in water
- Melting Point 152-155 °C
- Formula C6H6O4
- Boiling Point 401.7 °C at 760 mmHg
- Molecular Weight 142.111
- Flash Point 179.9 °C
- Transport Information
- Appearance Tan powder
- Safety 22-24/25-36/37-36
- Risk Codes 40-68
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Kojic acid(whitening agent in cosmetic);3-O-Ethyl ascorbic acid;5-Hydroxy-2-hydroxymethyl-4H-4-pyranone;4H-Pyran-4-one,5-hydroxy-2-(hydroxymethyl)-;5-Hydroxy-2-hydroxymethyl-4-pyrone;5-Hydroxy-2-(hydroxymethyl)-4-pyrone;5-18-02-00516 (Beilstein Handbook Reference);5-hydroxy-2-(hydroxymethyl)pyran-4-one;5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one;2-(Hydroxymethyl)-5-hydroxy-4H-pyran-4-one;4H-Pyran-4-one, 5-hydroxy-2- (hydroxymethyl)-;
- PSA 70.67000
- LogP -0.16230
Synthetic route
-
-
26209-93-8
5-acetoxy-2-acetoxymethyl-4H-pyran-4-one

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 1h; | 95% |
| With methanol; ammonia |
-
-
7559-81-1
chlorokojic acid

-
-
67-64-1
acetone

-
A
-
644-46-2
allomaltol

-
B
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
C
-
16065-34-2
iodokojic acid

-
D
-
3019-04-3
iodoacetone

| Conditions | Yield |
|---|---|
| With sodium iodide at 45℃; for 24h; Substitution; | A n/a B n/a C 78% D n/a |


-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| With silica gel In methanol at 60℃; for 3h; Inert atmosphere; | 70% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-keto-D-glucose With sodium acetate; acetic anhydride In N,N-dimethyl-formamide for 23h; Stage #2: With sodium methylate In methanol for 1h; | 20% |

| Conditions | Yield |
|---|---|
| With Aspergillus oryzae Bei der Vergaerung; |

| Conditions | Yield |
|---|---|
| bei der Einw.verschiedener Essigbakterien; |

| Conditions | Yield |
|---|---|
| durch Aspergillus oryzae; |

| Conditions | Yield |
|---|---|
| bei der Einw.von Aspergillus oryzae; |

| Conditions | Yield |
|---|---|
| With Aspergillus oryzae Bei der Vergaerung; |

| Conditions | Yield |
|---|---|
| With aspergillus glaucus Bei der Vergaerung; | |
| bei der Einw.von Aspergillus glaucus; |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen under 258.776 Torr; for 0.166667h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: neat (no solvent) / 30 °C / Inert atmosphere; Schlenk technique; Glovebox 2: silica gel / methanol / 3 h / 60 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: ammonium hydroxide / 0.75 h / pH 6 / Enzymatic reaction 2.1: sodium acetate; acetic anhydride / N,N-dimethyl-formamide / 23 h 2.2: 1 h View Scheme |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
124-63-0
methanesulfonyl chloride

-
-
5443-45-8
5-methanesulfonyloxy-2-methanesulfonyloxymethyl-pyran-4-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 20℃; for 0.166667h; | 100% |
| With 1-chloro-1-(dimethylamino)-2-methyl-1-propene In dichloromethane for 3h; Inert atmosphere; chemoselective reaction; | 100% |
| With thionyl chloride at 20℃; for 3h; | 98% |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
2169-68-8
ethyl (E)-3-(4-chlorophenyl)-2-cyanoacrylate

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.166667h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.166667h; Product distribution; Heating; reaction with arylmethylenemalonitrile derivatives; | 99% |
| With piperidine In ethanol for 0.166667h; Heating; | 99% |
| With β‐cyclodextrin In water at 70℃; for 1h; Reagent/catalyst; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.166667h; Heating; | 99% |
| In water at 100℃; Michael Addition; Microwave irradiation; Sealed tube; Green chemistry; | 96% |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
2017-87-0
ethyl (2E)-2-cyano-3-(4-methoxyphenyl)acrylate

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.166667h; Heating; | 99% |
| With piperidine In ethanol for 4h; Cycloaddition; Heating; | 28% |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
100-39-0
benzyl bromide

-
-
15771-06-9
5-benzyloxy-2-hydroxymethyl-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 80℃; | 99% |
| With potassium carbonate In ethanol; water for 2h; Reflux; | 98% |
| With caesium carbonate In N,N-dimethyl-formamide at 70℃; for 0.5h; | 90% |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
108-05-4
vinyl acetate

-
-
25552-08-3
7-O-acetylkojic acid

| Conditions | Yield |
|---|---|
| novozyme 435 In acetonitrile at 50℃; Enzymatic reaction; | 99% |

| Conditions | Yield |
|---|---|
| In ethanol; chloroform at 20℃; for 1h; | 98.5% |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
824-94-2
p-methoxybenzyl chloride

-
-
118708-61-5
5-p-methoxybenzyloxy-2-hydroxymethyl-4-pyrone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 3h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 3h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; Reflux; | 90% |
-
-
37366-09-9
dichloro(benzene)ruthenium(II) dimer

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
1204169-40-3
(η6-C6H6)RuCl(5-hydroxy-2-(hydroxymethyl)-4-pyrone)

| Conditions | Yield |
|---|---|
| With NaOMe In methanol byproducts: NaCl; kojic acid and NaOMe in dry MeOH stirred for 10 min; Ru complex added, stirred for 5 h; rotary evapd. to dryness; taken in CH2Cl2; soln. filtered, concd. to 3 ml, then Et2O excess added; left for 2 h at room temp.; ppt. collected, washed with Et2O; dried in vac.; | 98% |
-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
620-23-5
m-tolyl aldehyde

-
-
109-77-3
malononitrile

-
-
876710-04-2
2-amino-6-(hydroxymethyl)-8-oxo-4-m-tolyl-4,8-dihydropyrano[3,2-b]pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene immobilized on silica In neat (no solvent) at 20℃; for 0.05h; | 98% |
| In water at 50℃; for 0.0833333h; Sonication; | 97% |
| With nanozeolite clinoptilolite In water at 95℃; for 0.333333h; Green chemistry; | 95% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
459-57-4
4-fluorobenzaldehyde

-
-
109-77-3
malononitrile

-
-
797028-50-3
2-amino-4-(4-fluorophenyl)-4,8-dihydro-6-(hydroxymethyl)-8-oxo-pyrano[3,2-b]pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| In water at 50℃; for 0.1h; Sonication; | 98% |
| With nanozeolite clinoptilolite In water at 95℃; for 0.25h; Green chemistry; | 96% |
| With nano-silica sulfuric acid In water for 0.416667h; Reflux; Green chemistry; | 95% |

-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
109-77-3
malononitrile

-
-
625376-09-2
2-amino-4-(pyridine-3-yl)-4,8-dihydro-6-(hydroxymethyl)-8-oxo-pyrano[3,2-b]pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| In water at 50℃; for 0.0833333h; Sonication; | 98% |
| With Ca9.5Mg0.5(PO4)5.5(SiO4)0.5F1.5 In ethanol for 0.833333h; Reflux; Green chemistry; | 85% |

-
-
98-01-1
furfural

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
109-77-3
malononitrile

-
-
873570-71-9
2-amino-4-(furan-2-yl)-6-(hydroxymethyl)-8-oxo-4,8-dihydropyrano[3,2-b]pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| In water at 50℃; for 0.0833333h; Sonication; | 98% |
| With nanozeolite clinoptilolite In water at 95℃; for 0.333333h; Green chemistry; | 97% |
| With β‐cyclodextrin In water at 70℃; for 1h; Green chemistry; | 93% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
104-88-1
4-chlorobenzaldehyde

-
-
109-77-3
malononitrile

| Conditions | Yield |
|---|---|
| With nanozeolite clinoptilolite In water at 95℃; for 0.3h; Green chemistry; | 98% |
| With nano Fe3O4(at) SiO2(at)BenzIm-Fc[Cl]/ZnCl2 In ethanol; water at 20℃; for 0.133333h; Sonication; | 98% |
| With sulfonic acid functionalized MCM-41 In water at 90℃; for 0.666667h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Green chemistry; | 97% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
104-87-0
4-methyl-benzaldehyde

-
-
109-77-3
malononitrile

| Conditions | Yield |
|---|---|
| With nano Fe3O4(at) SiO2(at)BenzIm-Fc[Cl]/ZnCl2 In ethanol; water at 20℃; for 0.133333h; Sonication; | 98% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene immobilized on silica In neat (no solvent) at 20℃; for 0.05h; | 97% |
| With ferrocene functionalized ionic liquid supported on silica coated Fe3O4 magnetic nanoparticles In ethanol; water at 25℃; for 0.166667h; Catalytic behavior; Solvent; Sonication; | 95% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
109-77-3
malononitrile

-
-
16588-34-4
4-chloro-3-nitro-benzaldehyde

-
-
1613234-40-4
2-amino-4-(4-chloro-3-nitrophenyl)-6-hydroxymethyl-8-oxo-4,8-dihydropyrano[3,2-b]pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| With nano-silica sulfuric acid In water for 0.2h; Reflux; Green chemistry; | 98% |
| With s-triazinium-based ionic liquid immobilized on silica-coated Fe3O4 magnetic nanoparticles In water at 100℃; for 0.5h; Green chemistry; | 98% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
105-07-7
4-cyanobenzaldehyde

-
-
109-77-3
malononitrile

-
-
1613234-41-5
2-amino-4-(4-cyanophenyl)-4,8-dihydro-6-(hydroxymethyl)-8-oxo-pyrano[3,2-b]pyran3-carbonitrile

| Conditions | Yield |
|---|---|
| With sulfonic acid functionalized MCM-41 In water at 90℃; for 0.583333h; Green chemistry; | 98% |
| With nano-silica sulfuric acid In water for 0.25h; Reflux; Green chemistry; | 94% |
| With Ca9.5Mg0.5(PO4)5.5(SiO4)0.5F1.5 In ethanol for 0.25h; Reflux; Green chemistry; | 92% |
| With β‐cyclodextrin In water at 70℃; for 1h; Green chemistry; | 90% |
| With lipase from Aspergillus niger In ethanol; water at 20℃; for 3.5h; Enzymatic reaction; | 90% |


| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In 1,4-dioxane; water at 20℃; for 24h; | 98% |
| With potassium modified alumina catalysts (CAT3) In 1,4-dioxane; water at 20℃; for 24h; Aldol Condensation; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| With s-triazinium-based ionic liquid immobilized on silica-coated Fe3O4 magnetic nanoparticles In water at 100℃; for 0.5h; Green chemistry; | 98% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
555-16-8
4-nitrobenzaldehdye

-
-
109-77-3
malononitrile

| Conditions | Yield |
|---|---|
| With s-triazinium-based ionic liquid immobilized on silica-coated Fe3O4 magnetic nanoparticles In water at 100℃; for 0.333333h; Green chemistry; | 98% |
| With sulfonic acid functionalized MCM-41 In water at 90℃; for 0.583333h; Green chemistry; | 98% |
| With C5H6NO3S(1+)*F6P(1-) In neat (no solvent) at 90℃; for 0.25h; Reagent/catalyst; | 92% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
50-00-0
formaldehyd

-
-
70931-28-1
1-(4-fluorophenylmethyl)piperazine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Mannich Aminomethylation; | 98% |

-
-
1121-60-4
pyridine-2-carbaldehyde

-
-
1824-81-3
2-Amino-6-methylpyridine

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| With hyper-cross-linked β-cyclodextrin nanosponge In ethanol for 0.25h; Solvent; Time; Mannich Aminomethylation; Reflux; | 98% |


| Conditions | Yield |
|---|---|
| Stage #1: piperazine; formaldehyd In ethanol at 20℃; for 0.5h; Stage #2: 5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one In ethanol for 5h; | 97% |
| 1) EtOH, H2O, 30 min, 2) EtOH, H2O, room temperature, 1 h; Yield given. Multistep reaction; |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
4021-50-5
4-(trifluoromethylthio)benzaldehyde

-
-
109-77-3
malononitrile

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.25h; Heating; | 97% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
123-62-6
propionic acid anhydride

-
-
1092474-58-2
2-propionyloxymethyl-4H-pyran-4-on-5-yl propionate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 97% |
-
-
120-72-9
indole

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
100-52-7
benzaldehyde

-
-
1261077-99-9
2-((1H-indol-3-yl)(phenyl)methyl)-3-hydroxy-6-(hydroxymethyl)-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one; benzaldehyde With FAU zeolite nanoparticles In neat (no solvent) at 110℃; for 0.166667h; Green chemistry; Stage #2: indole In neat (no solvent) Temperature; Green chemistry; | 97% |
| With Zn4O(BDC)3 supported on NiFe2O4 nanoparticles In neat (no solvent) at 110℃; for 1h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Concentration; | 92% |
| With indium(III) chloride at 120℃; for 1.08333h; neat (no solvent); | 85% |

-
-
98-03-3
thiophene-2-carbaldehyde

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
109-77-3
malononitrile

-
-
625376-15-0
2-amino-4-(thiophen-2-yl)-4,8-dihydro-6-(hydroxymethyl)-8-oxo-pyrano[3,2-b]pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| In water at 50℃; for 0.1h; Sonication; | 97% |
| With s-triazinium-based ionic liquid immobilized on silica-coated Fe3O4 magnetic nanoparticles In water at 100℃; for 0.416667h; Green chemistry; | 95% |
| With nanozeolite clinoptilolite In water at 95℃; for 0.3h; Green chemistry; | 92% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
91-56-5
indole-2,3-dione

-
-
105-56-6
ethyl 2-cyanoacetate

-
-
884213-99-4
ethyl 2'-amino-6'-(hydroxymethyl)-2,8'-dioxo-8'H-spiro[indoline-3,4'-pyrano[3,2-b]pyran]-3'-carboxylate

| Conditions | Yield |
|---|---|
| With N-doped graphene quantum dots modified with CuO (0D)/ZnO (1D) heterojunction In water for 0.25h; | 97% |
| With 1,4-diaza-bicyclo[2.2.2]octane In methanol at 60℃; for 12h; Catalytic behavior; Reagent/catalyst; Temperature; Time; Solvent; | 94% |
| With copper(II) bis(trifluoromethanesulfonate) In 1,2-dichloro-ethane for 0.583333h; Reflux; | 89% |

Kojic acid Chemical Properties
Molecular Formula: C6H6O4
Molecular Weight: 142.11
EINECS: 207-922-4
Index of Refraction: 1.606
Density: 1.542 g/cm3
Flash Point: 179.9 °C
Enthalpy of Vaporization: 75.44 kJ/mol
Boiling Point: 401.7 °C at 760 mmHg
Vapour Pressure: 4.01E-08 mmHg at 25 °C
Melting point: 152-155 °C
Water solubility: Soluble
Appearance: Tan powder
Structure of Kojic acid (CAS NO.501-30-4):
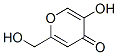
IUPAC Name: 5-Hydroxy-2-(hydroxymethyl)pyran-4-one
Product Category of Kojic acid (CAS NO.501-30-4): Heterocycles;Plant Oils, Toxins, Phenolic Acids & Derivatives
Kojic acid Uses
Kojic acid (CAS NO.501-30-4) can be used in food and cosmetics to preserve or change colors of substances. On cut fruits it is used to prevent oxidative browning, in seafood to preserve pink and red colors, and in cosmetics to lighten skin.
Kojic acid Toxicity Data With Reference
| 1. | mmo-sat 1 mg/plate | MUREAV Mutation Research. 67 (1979),367. | ||
| 2. | mma-sat 4 mg/plate | JTSCDR Journal of Toxicological Sciences. 7 (1982),255. | ||
| 3. | ivn-rat LDLo:1000 mg/kg | 85ERAY Antibiotics: Origin, Nature, and Properties. 3 (1978),1755. | ||
| 4. | orl-mus LDLo:4 g/kg | NATUAS Nature. 155 (1945),302. | ||
| 5. | ipr-mus LD50:250 mg/kg | 85GDA2 CRC Handbook of Antibiotic Compounds, Volumes 1-9. 5 (1981),393. | ||
| 6. | scu-mus LDLo:1500 mg/kg | NATUAS Nature. 155 (1945),302. | ||
| 7. | ivn-mus LDLo:1500 mg/kg | NATUAS Nature. 155 (1945),302. | ||
| 8. | ivn-dog LDLo:1000 mg/kg | 85ERAY Antibiotics: Origin, Nature, and Properties. 3 (1978),1755. | ||
| 9. | ivn-rbt LDLo:1000 mg/kg | 85ERAY Antibiotics: Origin, Nature, and Properties. 3 (1978),1755. |
Kojic acid Consensus Reports
EPA Genetic Toxicology Program.
Kojic acid Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion, intravenous, and subcutaneous routes. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes.
Hazard Codes:  Xn
Xn
Risk Statements:
R40:Limited evidence of a carcinogenic effect
R68 :Possible risk of irreversible effects
Safety Statements:
S22:Do not breathe dust
S24/25:Avoid contact with skin and eyes
S36/37:Wear suitable protective clothing and gloves
S36 :Wear suitable protective clothing
WGK Germany: 3
Kojic acid Specification
Kojic acid , its cas register number is 501-30-4. It also can be called 5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one ; and 5-Hydroxy-2-hydroxymethyl-4-pyrone . It is a mild inhibitor of the formation of pigment in plant and animal tissues. It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Then you have the ingesting of the product: Wash mouth out with water, and get medical aid immediately. Notes to physician: Treat supportively and symptomatically.
In addition, Kojic acid (CAS NO.501-30-4) is not compatible with strong oxidizing agents, and you must not take it with incompatible materials. And also prevent it to broken down into hazardous decomposition products: carbon monoxide, carbon dioxide.
Related Products
- Kojic acid
- Kojic acid dipalmitate
- 501325-89-9
- 501332-69-0
- 50-13-5
- 501357-89-7
- 501-36-0
- 5013-77-4
- 501433-05-2
- 501435-91-2
- 50-14-6
- 501-52-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View