-
Name
Pefloxacin
- EINECS 274-611-8
- CAS No. 70458-92-3
- Article Data13
- CAS DataBase
- Density 1.32 g/cm3
- Solubility
- Melting Point 270-272° (dec)
- Formula C17H20FN3O3
- Boiling Point 529.1 °C at 760 mmHg
- Molecular Weight 333.363
- Flash Point 273.8 °C
- Transport Information
- Appearance
- Safety
- Risk Codes R51/53
-
Molecular Structure
-
Hazard Symbols
 N
N
- Synonyms 1-Ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid;
- PSA 65.78000
- LogP 1.61340
Synthetic route
-
-
109-01-3
1-methyl-piperazine

-
-
68077-26-9
7-chloro-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
A
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| In water for 13h; Heating; | A 90% B n/a |
| In dimethylsulfoxide-d6 at 120 - 130℃; | A 82 % Spectr. B 18 % Spectr. |

-
-
50-00-0
formaldehyd

-
-
70458-96-7
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With formic acid Heating; | 85% |

-
-
68077-26-9
7-chloro-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
-
22206-57-1
tetra-n-butylammoniumfluoride trihydrate

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| In water; acetonitrile | 85% |

-
-
109-01-3
1-methyl-piperazine

-
-
68077-26-9
7-chloro-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| at 130 - 140℃; for 5h; | 68% |
-
-
70458-96-7
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid

-
-
74-88-4
methyl iodide

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 80 - 90℃; for 2h; | 30% |
-
-
109-01-3
1-methyl-piperazine

-
-
70032-25-6
6,7-difluoro-1-ethyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; | |
| In dimethyl sulfoxide at 40℃; Rate constant; or over the mixed anhydrides with BF3 or BBr3 (facilitate the aromatic substitution); |
-
-
74-83-9
methyl bromide

-
-
70458-96-7
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 80 - 90℃; for 2h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) DMSO, 110 deg C, 2 h; 2.) reflux, 1 h; Yield given; |
-
-
367-21-5
3-chloro-4-fluorophenylamine

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 100 percent / 2 h / 120 - 130 °C 2: diphenyl ether / 1 h / Heating 3: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 4: 90 percent / 2N NaOH / 2 h / Heating 5: 66 percent / 5 h / 130 - 140 °C 6: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: 100 percent / 2 h / 120 - 130 °C 2: diphenyl ether / 1 h / Heating 3: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 4: 90 percent / 2N NaOH / 2 h / Heating 5: 66 percent / 5 h / 130 - 140 °C 6: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 100 percent / 2 h / 120 - 130 °C 2: diphenyl ether / 1 h / Heating 3: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 4: 90 percent / 2N NaOH / 2 h / Heating 5: 68 percent / 5 h / 130 - 140 °C View Scheme |
-
-
68077-26-9
7-chloro-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 66 percent / 5 h / 130 - 140 °C 2: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 66 percent / 5 h / 130 - 140 °C 2: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme |
-
-
70458-94-5
1-ethyl-6-fluoro-7-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / 2N NaOH / 2 h / Heating 2: 66 percent / 5 h / 130 - 140 °C 3: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 90 percent / 2N NaOH / 2 h / Heating 2: 66 percent / 5 h / 130 - 140 °C 3: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 90 percent / 2N NaOH / 2 h / Heating 2: 68 percent / 5 h / 130 - 140 °C View Scheme |
-
-
74011-47-5
ethyl 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2N NaOH / 2 h / Heating 2: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 2N NaOH / 2 h / Heating 2: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme |
-
-
70032-30-3
diethyl 2-[(3-chloro-4-fluorophenylamino)methylene]malonate

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: diphenyl ether / 1 h / Heating 2: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 3: 90 percent / 2N NaOH / 2 h / Heating 4: 66 percent / 5 h / 130 - 140 °C 5: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: diphenyl ether / 1 h / Heating 2: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 3: 90 percent / 2N NaOH / 2 h / Heating 4: 66 percent / 5 h / 130 - 140 °C 5: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme | |
| Multi-step reaction with 4 steps 1: diphenyl ether / 1 h / Heating 2: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 3: 90 percent / 2N NaOH / 2 h / Heating 4: 68 percent / 5 h / 130 - 140 °C View Scheme |
-
-
70458-93-4
7-Chloro-6-fluoro-4-hydroxy-3-quinolinecarboxylic acid ethyl ester

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 2: 90 percent / 2N NaOH / 2 h / Heating 3: 66 percent / 5 h / 130 - 140 °C 4: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 2: 90 percent / 2N NaOH / 2 h / Heating 3: 66 percent / 5 h / 130 - 140 °C 4: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 90 percent / K2CO3 / dimethylformamide / 10 h / 80 - 90 °C 2: 90 percent / 2N NaOH / 2 h / Heating 3: 68 percent / 5 h / 130 - 140 °C View Scheme |
-
-
75001-56-8
6-Fluoro-4-hydroxy-7-piperazin-1-yl-quinoline-3-carboxylic acid ethyl ester

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: K2CO3 / dimethylformamide / 2 h / 60 - 90 °C 2: 2N NaOH / 2 h / Heating 3: 85 percent / 87percent HCOOH / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: K2CO3 / dimethylformamide / 2 h / 60 - 90 °C 2: 2N NaOH / 2 h / Heating 3: 30 percent / Et3N / dimethylformamide / 2 h / 80 - 90 °C View Scheme |
-
-
136491-15-1
N-ethyl-3,4-difluoroaniline

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 62 percent / 0.5 h / 90 - 100 °C 2: 71 percent / PPA / toluene / 0.58 h / 100 °C 3: dimethylsulfoxide / 40 °C / or over the mixed anhydrides with BF3 or BBr3 (facilitate the aromatic substitution) View Scheme |
-
-
136491-16-2
N-(3,4-Difluorphenyl)-N-ethyl-formamid

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 77 percent / conc. HCl / H2O / 2 h / Heating 2: 62 percent / 0.5 h / 90 - 100 °C 3: 71 percent / PPA / toluene / 0.58 h / 100 °C 4: dimethylsulfoxide / 40 °C / or over the mixed anhydrides with BF3 or BBr3 (facilitate the aromatic substitution) View Scheme |
-
-
136491-17-3
N-(3,4-Difluorphenyl)-N-ethyl-aminomethylen-meldrumsaeure

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / PPA / toluene / 0.58 h / 100 °C 2: dimethylsulfoxide / 40 °C / or over the mixed anhydrides with BF3 or BBr3 (facilitate the aromatic substitution) View Scheme |
-
-
3863-11-4
3,4-difluoroaniline

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 72.5 percent / conc. H2SO4 / 1.5 h / 175 °C 2: 77 percent / conc. HCl / H2O / 2 h / Heating 3: 62 percent / 0.5 h / 90 - 100 °C 4: 71 percent / PPA / toluene / 0.58 h / 100 °C 5: dimethylsulfoxide / 40 °C / or over the mixed anhydrides with BF3 or BBr3 (facilitate the aromatic substitution) View Scheme |
-
-
109-01-3
1-methyl-piperazine

-
-
68077-26-9
7-chloro-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
-
7440-44-0
pyrographite

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| In acetic acid; dimethyl sulfoxide |


-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With ammonia In water |

| Conditions | Yield |
|---|---|
| In methanol for 24h; Reflux; | 91% |


| Conditions | Yield |
|---|---|
| In methanol for 24h; Reflux; | 86% |


| Conditions | Yield |
|---|---|
| Stage #1: Pefloxacin With potassium hydroxide In methanol for 0.5h; Stage #2: methanol; 1,10-Phenanthroline; copper(II) choride dihydrate for 0.333333h; | 85% |

-
-
70458-92-3
Pefloxacin

-
-
530-62-1
1,1'-carbonyldiimidazole

| Conditions | Yield |
|---|---|
| In acetonitrile Solvent; Reflux; | 83.5% |
-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
-
70458-92-3
Pefloxacin

-
-
1368704-55-5
pefloxancindium tetrabromidozincate

| Conditions | Yield |
|---|---|
| In hydrogen bromide aq. HBr; pefloxacin dissolved in 6 M HBr; ZnO was slowly added to molar ratio Zn:pefloxacin = 2:1; solvent evapd.; crystals were obtained; elem. anal.; | 83% |


| Conditions | Yield |
|---|---|
| In methanol for 24h; Reflux; | 82% |


| Conditions | Yield |
|---|---|
| In methanol for 24h; Reflux; | 80% |

-
-
70458-92-3
Pefloxacin

-
-
110719-52-3
1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-2,3-dihydroquinolin-4(1Η)-one

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; toluene-4-sulfonic acid In methanol at 65℃; for 0.5h; | 76.2% |
| Stage #1: Pefloxacin With methanol; sodium tetrahydroborate at 0℃; for 1h; Stage #2: With toluene-4-sulfonic acid at 60 - 65℃; for 0.5h; | 76.3% |
-
-
10049-16-8
vanadium(IV) fluoride

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| In methanol methanolic soln. of VF4 added to methanolic soln. of C17H22O3N3F dropwise with stirring, mixt. had pH 3, refluxed for 2 h, kept overnight with stirring at room temp.; solvent removed on a rotary apparatus under reduced pressure; recrystn. (MeOH/n-hexane, 1/1); elem. anal.; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: Pefloxacin With potassium hydroxide In methanol for 0.5h; Stage #2: methanol; copper(II) choride dihydrate for 0.5h; | 75% |


| Conditions | Yield |
|---|---|
| Stage #1: Pefloxacin With potassium hydroxide In methanol for 0.5h; Stage #2: methanol; [2,2]bipyridinyl; copper(II) choride dihydrate for 0.333333h; | 75% |


| Conditions | Yield |
|---|---|
| In methanol for 24h; Reflux; | 74% |


| Conditions | Yield |
|---|---|
| With sulfuric acid for 12h; Reflux; | 73% |
-
-
1202-34-2
di(pyridin-2-yl)amine

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| Stage #1: Pefloxacin With potassium hydroxide In methanol for 0.5h; Stage #2: di(pyridin-2-yl)amine; copper(II) choride dihydrate In methanol for 0.333333h; | 70% |


| Conditions | Yield |
|---|---|
| With CH3ONa In methanol CH3OH soln. of Cu salt added to CH3OH soln. of bipyridyl amine (1:1), treated with 1 equiv. of levofloxacin in CH3OH, pH adjusted to 6.2 (CH3ONa), refluxed for 1 h; concd., washed (cold CH3OH, ether), dried (vac.), elem. anal.; | 68% |


| Conditions | Yield |
|---|---|
| With CH3ONa In methanol CH3OH soln. of Cu salt added to CH3OH soln. of bipyridyl amine (1:1), treated with 1 equiv. of pefloxacin in CH3OH, pH adjusted to 6.2 (CH3ONa),refluxed for 1 h; concd., washed (cold CH3OH, ether), dried (vac.), elem. anal.; | 63% |


-
-
7732-18-5
water

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 3h; pH=Ca. 6.8; Reflux; | 61.2% |

-
-
30050-39-6, 23311-38-8
[tris(triphenylphosphine) ruthenium(III) trichloride]

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; toluene Reflux; | 57.9% |

-
-
70458-92-3
Pefloxacin

-
-
2848-01-3
3-(diphenylphosphino)propionic acid

| Conditions | Yield |
|---|---|
| In methanol for 10h; pH=Ca. 6.8; Reflux; | 57.32% |

-
-
30050-39-6, 23311-38-8
[tris(triphenylphosphine) ruthenium(III) trichloride]

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With sodium methylate; triethylamine; lithium chloride In methanol; toluene Reflux; | 53.8% |

| Conditions | Yield |
|---|---|
| In water at 80℃; | 53% |

-
-
70458-92-3
Pefloxacin

-
-
2848-01-3
3-(diphenylphosphino)propionic acid

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 3h; pH=Ca. 6.8; Reflux; | 52.3% |


| Conditions | Yield |
|---|---|
| With water at 80℃; | 43% |
-
-
1215036-45-5
3-(1-amino-5-mercapto-1H-[1,3,4]triazol-2-yl)-1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4(1H)quinolinone

-
-
70458-92-3
Pefloxacin

| Conditions | Yield |
|---|---|
| With dmap; trichlorophosphate at 20℃; Reflux; | 34% |
Pefloxacin Chemical Properties
Pefloxacin(70458-92-3) is a synthetic chemotherapeutic agent used to treat severe and life threatening bacterial infections. Pefloxacin is commonly referred to as a fluoroquinolone (or quinolone) drug and is a member of the fluoroquinolone class of antibacterials. It is an analog of norfloxacin. It is a synthetic fluoroquinolone, belonging to the 3rd generation of quinolones. Pefloxacin is extensively prescribed in France. Pefloxacin has not been approved for use in the United States.
There are no licensed uses for Pefloxacin(70458-92-3) in the United States, as the FDA has not approved this drug. The licensed use varies in other countries and is quite limited as Pefloxacin is to be considered a drug of last resort when all other antibiotics have failed. There appears to be eight common uses in the adult population and no approved uses in the pediatric population, as well as a variety of veterinary uses (as documented within the package inserts). Pefloxacin interacts with a number of other drugs, a number of herbal and natural supplements, and certain thyroid medications.
The molecular formula of Pefloxacin mesylate(70458-92-3) is C17H20FN3O3 and its formula weight is 465.49.
The chemical synonyms of Pefloxacin mesylate(70458-92-3) are RB-1589;PEFLOXACIN;PEFLOXACINE MESYLATE DIHYDRATE;PEFLOXACINE METHANSULFONATE DIHYDRATE;PEFLOXACIN MESYLATE DIHYDRATE;PEFLOXACIN METHANESULFONATE;PEFLOXACIN METHANE SULFONATE DIHYDRATE;PERFLOXACINE
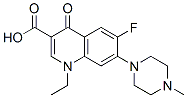
The molecular structure of Pefloxacin mesylate(70458-92-3):
Pefloxacin Uses
Pefloxacin has been increasingly used as a veterinary medicine to treat microbial infections.
Antibiotics such as Pefloxacin do not improve sinusitis symptoms. When prescribed for Community Acquired Pneumonia, Chronic Bronchitis, and Acute Bacterial Sinusitis the use of the fluoroquinolone class offers no compelling advantages over established treatment Nor does antibiotic treatment help sore throats. The use of antibiotics such as Pefloxacin to treat bronchitis is to be considered unnecessary and as such exposes the patient to an unacceptable risk of suffering a severe adverse reaction. Antibiotics' futility against bronchitis had been confirmed in 2002. Since Streptococci and Pneumococci show only intermediate susceptibility to pefloxacin, the drug should not be prescribed as 1st-line treatment in respiratory tract infections, when bacteriological examination has not been carried out.
Additionally Pefloxacin and other fluoroquinolones have no effect upon viral infections such as the common head cold.
Pefloxacin Specification
Pefloxacin should only be administered as described within the Dosage Guidelines table found within the most current package insert. The status of the patient’s renal function and hepatic function must also be taken into consideration to avoid an accumulation that may lead to a fatal drug overdose. Pefloxacin is eliminated partially through renal excretion. However, the drug is also metabolized and partially cleared through the liver and the intestine. Modification of the dosage is recommended using the table found within the package insert for those with impaired liver or kidney function. (Particularly for patients with severe renal dysfunction.) However, since the drug is known to be partially excreted by the kidneys, the risk of toxic reactions to this drug may be greater in patients with impaired renal function.
Recommended Doseage: 400 mg p.o. BID or QD Injectable; Injection; Pefloxacin Mesylate Dihydrate 400 mg / 5 ml Tablet, Film-Coated; Oral; Pefloxacin Mesylate Dihydrate 400 mg Oral Tablets: 400 mg Twice daily Injection: Administer by slow I.V. at a dosage of 400 mg diluted in 100 or 250 ml of 5% isotonic solution (Over a period of 1 hr) Twice daily.
Related Products
- Pefloxacin
- Pefloxacin mesylate
- 70458-95-6
- 70458-96-7
- 70460-50-3
- 70460-63-8
- 70464-36-7
- 70465-61-1
- 70468-44-9
- 70-47-3
- 70476-82-3
- 70476-91-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View