-
Name
Temozolomide
- EINECS 630-358-9
- CAS No. 85622-93-1
- Article Data33
- CAS DataBase
- Density 1.97 g/cm3
- Solubility
- Melting Point 212 °C dec.
- Formula C6H6N6O2
- Boiling Point 526.6 °C at 760 mmHg
- Molecular Weight 194.153
- Flash Point 272.3 °C
- Transport Information
- Appearance Off-white to light-pink crystalline solid
- Safety 24/25-45-36/37-26-53
- Risk Codes 45-46-60-61-22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T,
T,  Xi
Xi
- Synonyms 3,4-Dihydro-3-methyl-4-oxoimidazo(5,1-d)-as-tetrazine-8-carboxamide;CCRG 81045;Temozolodida [Spanish];Imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide, 3, 4-dihydro-3-methyl-4-oxo-;Imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide,3,4-dihydro-3-methyl-4-oxo-;3-methyl-2-oxo-1,3,4,5,8-pentazabicyclo[4.3.0]nona-4,6,8-triene-7-carboxamide;MB 39831;Methazolastone;Temozolomide [BAN:INN];Imidazo(5,1-d)(1,2,3,5)tetrazine-8-carboxamide, 3,4-dihydro-3-methyl-4-oxo-;Temodal;3-Methyl-4-oxo-3,4-dihydroimidazo(5,1-d)(1,2,3,5)tetrazine-8-carboxamide;Temozolomidum [Latin];8-Carbamoyl-3-methylimidazo(5,1-d)-1,2,3,5-tetrazin-4(3H)-one;
- PSA 108.17000
- LogP -1.37780
Synthetic route

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 480h; | 98% |
| In 1,4-dioxane at 50℃; | 76.5% |
| In dimethyl sulfoxide at 25℃; |

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(II) sulfate at 100℃; for 6h; Reagent/catalyst; Temperature; | 92% |

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With Lewatit Mono Plus MP-64 In acetone at 60 - 63℃; styrene-divinylbenzene (macroporous); Purification / work up; | 90.3% |
| With acetic acid In water; acetone for 0.166667h; Purification / work up; Heating / reflux; | 88% |
| With acetic acid In water; acetonitrile at 60 - 63℃; for 0.166667h; Purification / work up; | 86.7% |
| With acetic acid In tetrahydrofuran; water for 0.166667h; Purification / work up; Heating / reflux; | 70% |

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| In methanol; acetone at 20℃; for 1h; UV-irradiation; | 79% |

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In acetic acid; acetonitrile at 25℃; for 24h; | 78% |
| With tetrabutyl ammonium fluoride; acetic acid In acetonitrile at 25℃; | 78% |
-
-
188612-53-5
5-amino-N1-methyl-1H-imidazole-1,4-dicarboxamide

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Stage #1: 5-amino-N1-methyl-1H-imidazole-1,4-dicarboxamide With lithium chloride hydrate; acetic acid In water at 20℃; for 0.5h; Stage #2: With iodine; sodium nitrite In water at -10 - 20℃; for 3h; | 76% |
| Stage #1: 5-amino-N1-methyl-1H-imidazole-1,4-dicarboxamide With acetic acid; lithium bromide In water at 20℃; for 1h; Stage #2: With sodium nitrite In water at -10 - 20℃; for 6h; Stage #3: With sodium thiosulfate In water for 0.333333h; Product distribution / selectivity; | 64% |
| Stage #1: 5-amino-N1-methyl-1H-imidazole-1,4-dicarboxamide With sodium nitrite In water at 0℃; for 0.333333h; Stage #2: With tartaric acid In water at 0 - 60℃; for 4h; | 47% |
-
-
442534-20-5
N-tert-butyl-3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With sulfuric acid at 15 - 20℃; for 3h; | 52% |
-
-
188612-53-5
5-amino-N1-methyl-1H-imidazole-1,4-dicarboxamide

-
A
-
85622-93-1
temozolomide

-
B
-
4656-86-4
2-Azahypoxanthine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at 5 - 25℃; | A 35% B 15% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile | 22% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 25℃; | 22% |
-
-
157467-00-0
(8-Carbamoyl-4-oxo-imidazo[5,1-d][1,2,3,5]tetrazin-3-yl)-acetic acid 2-thioxo-2H-pyridin-1-yl ester

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In N,N-dimethyl-formamide at 25℃; Irradiation; Yield given; | |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In N,N-dimethyl-formamide for 0.5h; Ambient temperature; Irradiation; Yield given; |
-
-
77-78-1
dimethyl sulfate

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With triethylamine In dimethyl sulfoxide at 25℃; Yield given; |
-
-
188612-53-5
5-amino-N1-methyl-1H-imidazole-1,4-dicarboxamide

-
A
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With tartaric acid; sodium nitrite In water at 0℃; for 1h; | A 45 % Chromat. B n/a |

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 74 percent / aq. HCl / acetic acid / 0.42 h / Heating 2: 79 percent / acetone; methanol / 1 h / 20 °C / UV-irradiation View Scheme |
-
-
1025840-09-8
C17H18N6O3

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2.05 g / TFA / acetic acid; methanol / 5 h / 20 °C 2: 74 percent / aq. HCl / acetic acid / 0.42 h / Heating 3: 79 percent / acetone; methanol / 1 h / 20 °C / UV-irradiation View Scheme |
-
-
196806-10-7
5-Amino-4-carbamoyl-imidazole-1-carboxylic acid 4-nitro-phenyl ester

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 48 percent / tetrahydrofuran; ethanol / 25 °C 2: 45 percent Chromat. / NaNO2, tartaric acid / H2O / 1 h / 0 °C View Scheme |
-
-
157466-98-3
8-carbamoyl-3,4-dihydro-4-oxoimidazo<5,1-d>-1,2,3,5-tetrazin-3-ylacetic acid

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-methylmorpholine / dimethylformamide / 0.17 h / -15 - -10 °C 2: Et3N / dimethylformamide / 0.5 h / -15 - -10 °C 3: AIBN, Bu3SnH / dimethylformamide / 0.5 h / Ambient temperature; Irradiation View Scheme | |
| Multi-step reaction with 3 steps 1: N-methylmorpholine / dimethylformamide / -15 °C 2: Et3N / -15 °C 3: Bu3SnH, AIBN / dimethylformamide / 25 °C / Irradiation View Scheme |
-
-
157466-97-2
ethyl (8-carbamoyl-3,4-dihydro-4-oxoimidazo<5,1-d>-1,2,3,5-tetrazin-3-yl)acetate

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 80 percent / aq. HCl / 4 h / 40 - 45 °C 2: N-methylmorpholine / dimethylformamide / 0.17 h / -15 - -10 °C 3: Et3N / dimethylformamide / 0.5 h / -15 - -10 °C 4: AIBN, Bu3SnH / dimethylformamide / 0.5 h / Ambient temperature; Irradiation View Scheme | |
| Multi-step reaction with 4 steps 1: 80 percent / 5M aq. HCl / 45 °C 2: N-methylmorpholine / dimethylformamide / -15 °C 3: Et3N / -15 °C 4: Bu3SnH, AIBN / dimethylformamide / 25 °C / Irradiation View Scheme |
-
-
157466-99-4
C12H14N6O6

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Et3N / dimethylformamide / 0.5 h / -15 - -10 °C 2: AIBN, Bu3SnH / dimethylformamide / 0.5 h / Ambient temperature; Irradiation View Scheme | |
| Multi-step reaction with 2 steps 1: Et3N / -15 °C 2: Bu3SnH, AIBN / dimethylformamide / 25 °C / Irradiation View Scheme |
-
-
157466-96-1
[(5-Amino-4-carbamoyl-imidazole-1-carbonyl)-amino]-acetic acid ethyl ester

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 93 percent / 2N aq. NCl, NaNO2 / 0 °C 2: 80 percent / 5M aq. HCl / 45 °C 3: N-methylmorpholine / dimethylformamide / -15 °C 4: Et3N / -15 °C 5: Bu3SnH, AIBN / dimethylformamide / 25 °C / Irradiation View Scheme |
-
-
72-40-2
5-amino-1H-imidazole-4-carboxamide monohydrochloride

-
-
7693-46-1
4-Nitrophenyl chloroformate

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane |

-
-
74-88-4
methyl iodide

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Stage #1: 4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.05h; Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 2.5h; | |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 5h; |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 72h; Industry scale; |
-
-
1332700-58-9
tert-butyl (8-carbamoyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazin-3(4H)-yl)methylcarbamate

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water / 15 h / 4 - 20 °C / Darkness 2: sodium hydride / N,N-dimethyl-formamide / 5 h / 0 - 20 °C View Scheme |

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: boron trifluoride diethyl etherate / chloroform / 2 h / 0 - 20 °C 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile View Scheme | |
| Multi-step reaction with 2 steps 1: boron trifluoride / chloroform / 0 °C 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile / 25 °C View Scheme |
-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: acetonitrile; N,N-dimethyl-formamide / 2 h / 20 °C 2.1: acetonitrile / 6 h / 55 - 60 °C 3.1: sodium nitrite / water / 0.33 h / 0 °C 3.2: 4 h / 0 - 60 °C View Scheme |
-
-
85622-93-1
temozolomide

-
-
113942-30-6
3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrahydro-8-carboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite | 100% |
| With sulfuric acid; water; sodium nitrite at 15 - 20℃; | 98.6% |
| With sulfuric acid; sodium nitrite In water at 0℃; | 80% |

-
-
85622-93-1
temozolomide

-
-
21157-24-4
methyl (Z)-2-((3R,4S,5S,8S,9S,10S,11R,13R,14S,16S)-16-acetoxy-3,11-dihydroxy-4,8,10,14-tetramethylhexadecahydro-17H-cyclopenta[a]phenanthren-17-ylidene)-6-methylhept-5-enoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 97% |
-
-
85622-93-1
temozolomide

-
-
144701-48-4
telmisatran

-
-
528560-93-2
4'-[[4-methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl]biphenyl-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 95% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 95% |

| Conditions | Yield |
|---|---|
| In ethanol for 1h; | 94% |
-
-
53-86-1
[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetic acid

-
-
85622-93-1
temozolomide

-
-
1601-18-9
indomethacin methyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With meso-tetraphenylporphyrin iron(III) chloride; potassium hydroxide In water at 23℃; for 4h; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With meso-tetraphenylporphyrin iron(III) chloride; potassium hydroxide In water at 23℃; for 4h; Catalytic behavior; Reagent/catalyst; Solvent; Sealed tube; | 91% |
-
-
29584-19-8, 471-80-7
steviol

-
-
85622-93-1
temozolomide

-
-
14364-16-0
methyl (4R,4aS,6aR,9S,11aR,11bS)-9-hydroxy-4,11b-dimethyl-8-methylenetetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 90% |
-
-
110299-94-0
4-isopropylidene cyclohex-1-enyl methanol

-
-
85622-93-1
temozolomide

| Conditions | Yield |
|---|---|
| Stage #1: temozolomide With oxalyl dichloride In 1,2-dichloro-ethane at 10℃; for 3h; Swern Oxidation; Inert atmosphere; Reflux; Stage #2: 4-isopropylidene cyclohex-1-enyl methanol In 1,2-dichloro-ethane at 20℃; for 14h; Time; Reflux; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: oxalyl dichloride; temozolomide In 1,2-dichloro-ethane at 20℃; for 3h; Inert atmosphere; Stage #2: (-)-perillyl alcohol In 1,2-dichloro-ethane at 20℃; for 14h; Inert atmosphere; | 89% |

-
-
302-97-6
androst-4-en-3-one-17β-carboxylic acid

-
-
85622-93-1
temozolomide

-
-
2681-55-2
3-oxo-androst-4-ene-17β-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 4h; Sealed tube; | 89% |
-
-
619-84-1
p-N,N-dimethylaminobenzoic acid

-
-
85622-93-1
temozolomide

-
-
1202-25-1
methyl 4-(N,N-dimethylamino)benzoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 88% |
-
-
85622-93-1
temozolomide

-
-
181955-79-3
N,N'-di-tert-butyloxycarbonylpiperazine-2-carboxylic acid

-
-
171504-98-6
O1,O4-di-tert-butyl O2-methyl piperazine-1,2,4-tricarboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 88% |
-
-
53716-49-7
2-(6-chloro-carbazol-2-yl)-propionic acid

-
-
85622-93-1
temozolomide

-
-
52263-88-4
methyl (2RS)-2-(6-chloro-9H-carbazol-2-yl)propanoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 88% |
-
-
35661-40-6
N-Fmoc L-Phe

-
-
85622-93-1
temozolomide

-
-
129397-81-5
methyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalaninate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 87% |

| Conditions | Yield |
|---|---|
| With meso-tetraphenylporphyrin iron(III) chloride; potassium hydroxide In water at 23℃; for 4h; Sealed tube; | 87% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 86% |
-
-
5937-49-5
epigibberic acid

-
-
85622-93-1
temozolomide

-
-
43207-02-9
(4bR,7S,9aS,10R)-1,7-dimethyl-8-oxo-4b,6,7,8,9,10-hexahydro-5H-7,9a-methanobenzo[a]azulene-10-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 86% |
-
-
85622-93-1
temozolomide

-
-
143824-78-6
3-[(S)-2-carboxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)ethyl]indole-1-carboxylic acid tert-butyl ester

-
-
405555-31-9
tert-butyl (S)-3-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-methoxy-3-oxopropyl)-1H-indole-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 85% |
-
-
85622-93-1
temozolomide

-
-
301323-33-1
3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carbothioamide

| Conditions | Yield |
|---|---|
| With 2,4-(4-phenoxyphenyl)-1,3-dithia-2λ(5),4λ(5)-diphosphetane 2,4-disulfides In dichloromethane Reflux; | 84% |
| With Lawessons reagent In dichloromethane Reflux; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 83% |
-
-
104292-82-2
4-<(tetrahydropyran-2-yloxy)methyl>benzoic acid

-
-
85622-93-1
temozolomide

-
-
104292-97-9
methyl 4-<(tetrahydropyran-2-yloxy)methyl>benzoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 4h; Sealed tube; | 83% |
-
-
104-01-8
4-Methoxyphenylacetic acid

-
-
85622-93-1
temozolomide

-
-
23786-14-3
4-methoxy-phenyl acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 83% |
-
-
26171-23-3
1-methyl-5-(p-toluoyl)pyrrole-2-acetic acid

-
-
85622-93-1
temozolomide

-
-
33369-52-7
methyl 1-Methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 81% |
-
-
85622-93-1
temozolomide

-
-
510-50-9, 25274-38-8, 76675-42-8
(1R,2S,4aR,4bR,7S,9aS,10S)-2,7-dihydroxy-1-methyl-8-methylene-13-oxo-1,2,4b,5,6,7,8,9,10,10a-decahydro-4a,1-(epoxymethano)-7,9a-methanobenzo[a]azulene-10-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 81% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 60℃; for 6h; Sealed tube; | 80% |
Temozolomide Chemical Properties
IUPAC Name: 3-Methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide
Synonyms of Temozolomide (CAS NO.85622-93-1): 3,4-Dihydro-3-methyl-4-oxoimidazo(5,1-d)-1,2,3,5-tetrazine-8-carboxamide ; 3-Methyl-4-oxo-3,4-dihydroimidazo(5,1-d)(1,2,3,5)tetrazine-8-carboxamide ; 8-Carbamoyl-3-methylimidazo(5,1-d)-1,2,3,5-tetrazin-4(3H)-one
CAS NO: 85622-93-1
Molecular formula: C6H6N6O2
Molar weight: 194.15
Molecular Structure: 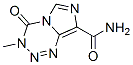
H bond acceptors: 8
H bond donors: 2
Freely Rotating Bonds: 1
Polar Surface Area: 83.16 Å2
Index of Refraction: 1.895
Molar Refractivity: 45.6 cm3
Molar Volume: 98.3 cm3
Surface Tension: 105 dyne/cm
Density: 1.97 g/cm3
Flash Point: 272.3 °C
Enthalpy of Vaporization: 80.1 kJ/mol
Boiling Point: 526.6 °C at 760 mmHg
Vapour Pressure: 3.53E-11 mmHg at 25°C
Storage temp: 2-8°C
Melting Point: 212°C dec.
Appearance: Off-White to Light-Pink Crystalline Solid
Product Categories of Temozolomide (CAS NO.85622-93-1): Active Pharmaceutical Ingredients;Antineoplastic;Intermediates & Fine Chemicals;Pharmaceuticals;Antitumour
Temozolomide Uses
Temozolomide (CAS NO.85622-93-1) can be used in treatment of Grade IV astrocytoma. Methylation damages the DNA and triggers the death of tumor cells,temozolomide's ability to alkylate/methylate DNA, which most often occurs at the N-7 or O-6 positions of guanine residues for the therapeutic benefit of temozolomide depends .
Temozolomide Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | intraperitoneal | 125mg/kg (125mg/kg) | Cancer Research. Vol. 47, Pg. 5846, 1987. |
Temozolomide Safety Profile
Safety Information about Temozolomide (CAS NO.85622-93-1):
Hazard Codes:  T,
T,  Xi
Xi
The Risk Statements: 45-46-60-61-22-36/37/38
45: May cause cancer
46: May cause heritable genetic damage
60: May impair fertility
61: May cause harm to the unborn child
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements: 24/25-45-36/37-26-53
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
53: Avoid exposure - obtain special instruction before use
24/25: Avoid contact with skin and eyes
36/37: Wear suitable protective clothing and gloves
Temozolomide Analytical Methods
Laboratory studies and clinical trials are investigating whether it might be possible to further increase the anticancer potency of temozolomide by combining it with other pharmacologic agents.
Related Products
- Temozolomide
- 85622-95-3
- 856245-74-4
- 856250-49-2
- 856250-60-7
- 85625-91-8
- 85631-66-9
- 85631-82-9
- 85631-84-1
- 85631-88-5
- 85631-89-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View