-
Name
Titanyl phthalocyanine
- EINECS 419-970-5
- CAS No. 26201-32-1
- Article Data24
- CAS DataBase
- Density
- Solubility
- Melting Point 395.1 °C (dec.)(lit.)
- Formula C32H16N8OTi
- Boiling Point
- Molecular Weight 576.412
- Flash Point
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Titanium,oxo[29H,31H-phthalocyaninato(2-)-N29,N30,N31,N32]-, (SP-5-12)-;29H,31H-Phthalocyanine, titanium complex;ELA 7051;Fastogen Blue 8310;Phthalocyaninetitanyl complex;T 22S;a-Titanyl phthalocyanine;
- PSA 101.09000
- LogP 1.34560
Synthetic route
-
-
1208497-30-6, 16903-42-7
dichloro(phthalocyaninato)titanium(IV)

-
-
7732-18-5
water

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

| Conditions | Yield |
|---|---|
| In pyridine Ti-complex was boiled in aq. pyridine for 2 h, cooled; filtered, washed with water, dried in vacuo at 100°C; elem. anal.; | 97% |
| With hydrogenchloride In DMF (N,N-dimethyl-formamide); water at 100 - 120℃; for 1h; |

| Conditions | Yield |
|---|---|
| In quinoline at 180℃; for 6h; Product distribution / selectivity; | 76.8% |
| With thiourea In further solvent(s) TiCl4 was mixed with heptanol, heated to boiling, ligand was added, withor without thiourea, heated to 170°C, kept for 6 h; cooled, filtered, washed with toluene and MeOH, extd. with MeOH in Soxhlet for 4 h, dried in air; elem. anal.; | 60% |
| In ethanol at -20.01℃; under 46504.7 Torr; for 1h; | 60% |
-
-
160956-25-2, 160956-26-3, 160956-27-4, 160956-28-5, 160956-29-6
isoindole-1,3-diylidenediamine

-
-
3087-37-4
titanium tetra-n-propoxide

-
-
91-15-6
phthalonitrile

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 198℃; for 2h; Product distribution / selectivity; Heating / reflux; | 76% |


-
-
160956-25-2, 160956-26-3, 160956-27-4, 160956-28-5, 160956-29-6
isoindole-1,3-diylidenediamine

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
B
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| In 1-chloronaphthalene (CINp) at 140 - 200℃; for 2h; Product distribution / selectivity; | A 72% B n/a |


| Conditions | Yield |
|---|---|
| In 1-Chloronaphthalene at 195℃; for 3h; Product distribution / selectivity; Heating / reflux; | 70% |
| In 1-methyl-pyrrolidin-2-one at 50 - 95℃; for 6h; Product distribution / selectivity; | 1.5% |
| In 1-methyl-pyrrolidin-2-one at 24 - 100℃; Product distribution / selectivity; Electrochemical reaction; | |
| In ethylene glycol at 24 - 100℃; Product distribution / selectivity; Electrochemical reaction; | |
| In sulfolane Inert atmosphere; |
-
-
1208497-30-6, 16903-42-7
dichlorotitanium phthalocyanine

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; N,N-dimethyl-formamide In water at 100 - 120℃; for 1h; pH=>= 6; |

| Conditions | Yield |
|---|---|
| With pentan-1-ol; benzamide In xylene at 144℃; for 6h; Heating / reflux; |
-
-
80937-33-3
oxygen

-
-
7550-45-0
titanium tetrachloride

-
-
91-15-6
phthalonitrile

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

| Conditions | Yield |
|---|---|
| In 1-Chloronaphthalene at 25 - 210℃; for 5h; | |
| In Diphenylmethane; 1,1,2,2-tetrachloroethane at 40 - 210℃; for 6 - 8h; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| With tetrabutoxytitanium In nonyl alcohol at 160 - 170℃; for 0.1 - 6h; Product distribution / selectivity; Microwave and ultrasonic energy; |
-
-
160956-25-2, 160956-26-3, 160956-27-4, 160956-28-5, 160956-29-6
isoindole-1,3-diylidenediamine

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

| Conditions | Yield |
|---|---|
| With quinoline; tetrabutoxytitanium at 170 - 180℃; for 0.1 - 6h; Product distribution / selectivity; |

-
-
139152-08-2
4,5-dichlorophthalonitrile

-
-
91-15-6
phthalonitrile

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

| Conditions | Yield |
|---|---|
| With benzamide In pentan-1-ol; xylene Reflux; |


| Conditions | Yield |
|---|---|
| With tin(IV) chloride In octanol at 155 - 159℃; for 6h; Inert atmosphere; | 22.77 g |

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
28178-42-9
2,6-diisopropylphenyl isocyanate

-
-
1276014-86-8
(2,6-diisopropylphenylimido)phthalocyaninatotitanium(IV)

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: CO2; under N2; mixt. of Ti complex and ((CH3)2CH)2C6H3NCO (20-fold excess) inchloronaphthalene heated at 180°C for 6 h, released CO2 replaced by N2 from time to time; mixt. cooled, pptn. by addn. of hexane, solid filtered off, extd. repeatedly with MeCN and toluene (10x50 mL each) under reflux, washed with pentane, dried at 120°C under vac. for 3 h; elem. anal.; | 83% |
-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
622-58-2
p-Tolylisocyanate

-
-
1217488-65-7
(N,N'-di(p-tolyl)ureato-κ2N,N')phthalocyaninatotitanium(IV)

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: CO2; under N2; mixt. of Ti complex and p-tolyl isocyanate in chloronaphthalene heated at 180°C for 6 h, released CO2 replaced by N2 from time to time; soln. cooled, pptn. by addn. of hexane, solid filtered off, extd. with rMeCN and toluene (10x50 mL each) under reflux, washed with pentane, dried at 120°C under vac. for 3 h; elem. anal.; | 76% |
| In further solvent(s) according to Darwish, W. et al., Acta Cryst. E61 2005, m1280-1282; Darwish, W., Dissertation, Philipps University, Marburg, Germany, 2006; solvent: chloronaphthalene; |

| Conditions | Yield |
|---|---|
| With fluorenone radical anion sodium contact ion pair In 1,2-dichloro-benzene at 80℃; for 24h; | 74% |

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: CO2; under N2; mixt. of Ti complex and mesityl isocyanate in chloronaphthalene heated at 180°C for 6 h, released CO2 replaced by N2 from time to time; soln. cooled, pptn. by addn. of hexane, solid filtered off, extd. with rMeCN and toluene (10x50 mL each) under reflux, washed with pentane, dried at 120°C under vac. for 3 h; elem. anal.; | 73% |
-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
4328-13-6
tetrahexylammonium bromide

-
-
95-50-1
1,2-dichloro-benzene

| Conditions | Yield |
|---|---|
| With fluorenone radical anion sodium contact ion pair at 80℃; for 24h; | 66% |

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
477-75-8
triptycene

-
-
4519-28-2
tetramethylphosphonium bromide

-
-
95-50-1
1,2-dichloro-benzene

| Conditions | Yield |
|---|---|
| Stage #1: oxotitanium(IV) phthalocyanine; tetramethylphosphonium bromide; 1,2-dichloro-benzene With fluorenone radical anion sodium contact ion pair at 100℃; for 2h; Glovebox; Inert atmosphere; Stage #2: triptycene In benzonitrile at 20 - 100℃; for 3h; | 65% |


| Conditions | Yield |
|---|---|
| With triphenylphosphine In further solvent(s) (N2); Schlenk techniques; phthalocyanine deriv. and P4S10 heated in chloronaphthalene at 160°C for 10 h; pptd. by addn. of hexane; washedby extn. with refluxing MeCN, toluene; washed with pentane; dried at 12 0.degre.C for 1 h under 1E-3 mbar; heated with triphenylphosphine to a melt at 160°C for 4 h; washedwith refluxing MeCN, toluene; washed with ether; dried at 120.degre.C f or 1 h under 1E-3 mbar; | 57% |
-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
14968-74-2
4-aza-1-methylazoniabicyclo<2.2.2>octane iodide

| Conditions | Yield |
|---|---|
| With fluorenone radical anion sodium contact ion pair In 1,2-dichloro-benzene at 100℃; for 24h; | 43% |
-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
14968-74-2
4-aza-1-methylazoniabicyclo<2.2.2>octane iodide

| Conditions | Yield |
|---|---|
| With fluorenone radical anion sodium contact ion pair In 1,2-dichloro-benzene at 100℃; for 24h; | 38% |

| Conditions | Yield |
|---|---|
| Stage #1: oxotitanium(IV) phthalocyanine; N-methyl-1,4-diazabicyclo<2.2.2>octanium iodide With fluorenone radical anion sodium contact ion pair In 1,2-dichloro-benzene at 100℃; for 24h; Stage #2: triptycene In benzonitrile; 1,2-dichloro-benzene at 20℃; for 4h; | 27% |

-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
1621-46-1, 17647-60-8, 98092-70-7, 517-28-2
haematoxylin

| Conditions | Yield |
|---|---|
| In further solvent(s) Ti-complex was dissolved in 1-chloronaphthalene at 180°C under N2, hematoxylin was added, the soln. was stirred for 15 min; filtd., hexane was added to induce pptn., ppt. was collected and recrystd. from 1-chloronaphthalene/MeOH; | 19.1% |
-
-
26201-32-1
oxotitanium(IV) phthalocyanine

-
-
63371-84-6
gallium phthalocyanine hydroxide


| Conditions | Yield |
|---|---|
| With hydrogenchloride; N,N-dimethyl-formamide In water at 100 - 120℃; for 1h; pH=>= 6; |
Titanyl phthalocyanine Chemical Properties
Following is the structure of Titanyl phthalocyanine (CAS NO.26201-32-1):
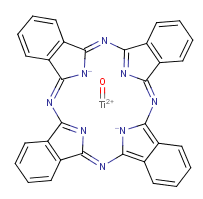
Empirical Formula: C32H16N8OTi
Molecular Weight: 576.3894
Melting point: 395 °C (dec.)
Product Categories: Organometallics; electronic;pharmacetical; Infrared (IR) DyesPhotonic and Optical Materials; Organic Electronics and Photonics; Photonic and Optical Materials; Phthalocyanine and Porphyrin Dyes; Phthalocyanines; Hole Transporting MaterialsOrganic Electronics and Photonics; OLED and PLED Materials
Canonical SMILES: C1=CC=C2C(=C1)C3=NC4=NC(=NC5=C6C=CC=CC6=C([N-]5)N=C7C8=CC=CC=C8C(=N7)N=C2[N-]3)C9=CC=CC=C94.O=[Ti+2]
InChI: InChI=1S/C32H16N8.O.Ti/c1-2-10-18-17(9-1)25-33-26(18)38-28-21-13-5-6-14-22(21)30(35-28)40-32-24-16-8-7-15-23(24)31(36-32)39-29-20-12-4-3-11-19(20)27(34-29)37-25;;/h1-16H;;/q-2;;+2
InChIKey: SJHHDDDGXWOYOE-UHFFFAOYSA-N
Titanyl phthalocyanine Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
TSCA: No
Titanyl phthalocyanine Specification
Titanyl phthalocyanine , its cas register number is 26201-32-1. It also can be called Titanium, oxo(29H,31H-phthalocyaninato(2-)-kappaN29,kappaN30,kappaN31,kappaN32)-, (SP-5-12)- . Its classification code is TSCA Flag P [A commenced PMN (Premanufacture Notice) substance].
Related Products
- Titanyl 2,4-pentanedionate
- Titanyl phthalocyanine
- 2620-27-1
- 26204-18-2
- 2620-44-2
- 26204-60-4
- 2620-50-0
- 2620-53-3
- 262-05-5
- 26206-00-8
- 2620-62-4
- 2620-63-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View