-
Name
tert-Butyl acrylate
- EINECS 216-768-7
- CAS No. 1663-39-4
- Article Data42
- CAS DataBase
- Density 0.883 g/cm3
- Solubility water solubility:2 g/L
- Melting Point -69 °C
- Formula C7H12O2
- Boiling Point 133 °C at 760 mmHg
- Molecular Weight 128.171
- Flash Point 31.6 °C
- Transport Information UN 1993 3/PG 2
- Appearance colorless liquid
- Safety 16-25-37-61
- Risk Codes 11-20/21/22-37/38-43-52/53
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xn
Xn
- Synonyms Acrylicacid, tert-butyl ester (6CI,8CI);1,1-Dimethylethyl 2-propenoate;2-Propenoicacid tert-butyl ester;NSC 20950;tert-Butyl 2-propenoate;tert-Butyl propenoate;
- PSA 26.30000
- LogP 1.51410
Synthetic route

| Conditions | Yield |
|---|---|
| With strong acid cation exchange resin; polymerization inhibitor A; polymerization inhibitor B at 10℃; for 13h; Temperature; | 99.7% |
| With tempol; 10H-phenothiazine; sulfuric acid | |
| With mesoporous Fe-SBA-15-SO3H at 60℃; for 12h; Autoclave; |
-
-
13831-03-3
tert-butyl prop-2-ynoate

-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With hydrogen; [Cp2Mo(μ-SH)2Rh(PPh3)2][PF6] In acetone at 20℃; under 760.051 Torr; for 5h; Product distribution; Further Variations:; Catalysts; Reaction partners; | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.25h; | 96% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; acetic anhydride at 0 - 60℃; for 5h; | 66% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide; Bu3PI2 In diethyl ether for 24h; | 51% |
| With nickel(II) polyacrylate; water |
-
-
292638-85-8
acrylic acid methyl ester

-
-
75-65-0
tert-butyl alcohol

-
A
-
1663-39-4
tert-Butyl acrylate

-
B
-
3852-09-3
methyl 3-methoxypropionate

-
C
-
81048-08-0
3-tert.-Butoxypropionsaeuremethylester

-
D
-
112032-54-9
3-Methoxy-propionic acid tert-butyl ester

-
E
-
21150-74-3
tert-butyl β-tert-butoxypropionate

| Conditions | Yield |
|---|---|
| With n-butyllithium; bis(acetylacetonate)nickel(II) In benzene at 90℃; for 5h; Product distribution; other time, other temp.; | A 33% B 26% C 9% D 32% E 3% |


| Conditions | Yield |
|---|---|
| With calcium carbonate at 70℃; | |
| With pyridine | |
| With triethylamine In chloroform at 10℃; for 12h; |

| Conditions | Yield |
|---|---|
| With 2,3-Dimethylaniline at 150℃; |
-
-
57-57-8
β-Propiolactone

-
-
74-88-4
methyl iodide

-
A
-
1663-39-4
tert-Butyl acrylate

-
B
-
6149-41-3
methyl ester (3-hydroxy) propionic acid

-
C
-
292638-85-8
acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium tert-butylate 1) THF, 20 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; tetracarbonyl nickel |


| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
548756-56-5
tert-butyl 3-hydroxy-3-(2-hydroxy-3-methoxyphenyl)-2-methylenenpropanoate

-
A
-
1663-39-4
tert-Butyl acrylate

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In chloroform-d1 retro-Baylis-Hillman reaction; |
-
-
548756-58-7
tert-butyl 3-(5-bromo-2-hydroxyphenyl)-3-hydroxy-2-methylenenpropanoate

-
A
-
1663-39-4
tert-Butyl acrylate

-
B
-
1761-61-1
5-bromosalicyclaldehyde

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In chloroform-d1 retro-Baylis-Hillman reaction; |
-
-
548756-57-6
tert-butyl 3-(3-ethoxy-2-hydroxyphenyl)-3-hydroxy-2-methylenenpropanoate

-
A
-
1663-39-4
tert-Butyl acrylate

-
B
-
492-88-6
3-ethoxysalicylaldehyde

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In chloroform-d1 retro-Baylis-Hillman reaction; |
-
-
548756-59-8
tert-butyl 3-(3,5-dibromo-2-hydroxyphenyl)-3-hydroxy-2-methylenenpropanoate

-
A
-
90-59-5
3,5-Dibromosalicylaldehyde

-
B
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In chloroform-d1 retro-Baylis-Hillman reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: acrolein With tert-butylhypochlorite In tetrachloromethane at 30℃; for 3.33333h; Stage #2: tert-butyl alcohol In tetrachloromethane at 50 - 75℃; for 22h; |
-
-
15026-17-2
succinic acid mono-tert-butyl ester

-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With 2,2-dimethylpropanoic anhydride; triphenylphosphine; palladium dichloride at 190℃; for 2h; Inert atmosphere; | 31 %Chromat. |
| Multi-step reaction with 2 steps 1.1: triethylamine / dichloromethane / 0.17 h / Inert atmosphere 1.2: 20 °C / Inert atmosphere 2.1: palladium dichloride; lithium chloride / 3 h / 155 - 160 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 2 steps 1: HATU; N-ethyl-N,N-diisopropylamine / dichloromethane / 4 h / 20 °C 2: triphenylphosphine / tetrahydrofuran; water / 20 °C View Scheme |

-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With lithium chloride; palladium dichloride at 155 - 160℃; for 3h; Inert atmosphere; Schlenk technique; Sealed tube; | 46 %Chromat. |

-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With triphenylphosphine In tetrahydrofuran; water at 20℃; |

-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrahydrofuran / 0 - 20 °C 2: Dess-Martin periodane / dichloromethane / 20 °C View Scheme |
-
-
548756-55-4
tert-butyl 3-hydroxy-3-(2-hydroxyphenyl)-2-methylenenpropanoate

-
A
-
1663-39-4
tert-Butyl acrylate

-
B
-
90-02-8
salicylaldehyde

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In [D3]acetonitrile at 20℃; Kinetics; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With 4-methoxy-phenol at 60℃; for 10h; |

| Conditions | Yield |
|---|---|
| Stage #1: acrylic acid With potassium carbonate In N,N-dimethyl-formamide at 0℃; for 0.75h; Inert atmosphere; Stage #2: t-butyl bromide In N,N-dimethyl-formamide at 100℃; for 24h; Inert atmosphere; |
-
-
1663-39-4
tert-Butyl acrylate

-
-
138034-79-4, 49855-41-6
tert-butyl 2,3-dibromopropanoate

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at 0 - 20℃; for 18h; | 100% |
| With bromine In dichloromethane at 5℃; for 2h; | 90% |
| With bromine In chloroform for 15h; Ambient temperature; | 81% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
208036-26-4
(E)-t-butyl 4-formylcinnamate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 6h; Heck-Mizoroki reaction; | 100% |
| With palladium diacetate; triethylamine; tris-(o-tolyl)phosphine Heating; | 91% |
| With palladium on silica; triethylamine In N,N-dimethyl-formamide for 0.2h; Heck Reaction; Microwave irradiation; Green chemistry; | 89% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
2357-39-3
2-trifluoromethyl-4-isopropyl-Δ3-oxazolin-5-one

-
-
87341-14-8
tert-butyl 3-[5-oxo-4-(propan-2-yl)-2-(trifluoromethyl)-2,5-dihydro-1,3-oxazol-2-yl]propanoate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| With triethylamine In dichloromethane 1.) -10 deg C, 2.) 25 deg C, 12 h; | 80% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
99-90-1
para-bromoacetophenone

-
-
389091-50-3
(E)-tert-butyl 3-(4-acetylphenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate; palladium diacetate In N,N-dimethyl-formamide at 120℃; for 15h; Heck cross-coupling reaction; | 100% |
| With 1,3-disubstituted imidazolium bromide; potassium carbonate; 4-methylmorpholine N-oxide; palladium diacetate In 1-methyl-pyrrolidin-2-one at 120℃; for 2h; Heck reaction; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 1h; Mizoroki-Heck reaction; | 99% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
829-85-6
diphenylphosphane

-
-
175407-89-3
3-Diphenylphosphanyl-propionic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With tetramethyl ammoniumhydroxide In tetrahydrofuran | 100% |
| In 2-methyltetrahydrofuran at 90℃; for 4h; Inert atmosphere; Sealed tube; Green chemistry; regioselective reaction; | 81% |
| In 2-methyltetrahydrofuran at 90℃; for 4h; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| With triethylamine; palladium In N,N-dimethyl acetamide at 100℃; for 24h; Heck reaction; | 100% |
| With C36H36Cl2N6Pd; triethylamine In methanol at 70℃; for 24h; Reagent/catalyst; Concentration; Heck Reaction; | 100% |
| With PdCl2(1-[2-(diphenylphosphanyl)ethyl]-3,5-dimethylpyrazole); tetrabutylammomium bromide; triethylamine In N,N-dimethyl-formamide at 140℃; for 0.16h; Catalytic behavior; Time; Heck Reaction; Inert atmosphere; Schlenk technique; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
624-31-7
4-tolyl iodide

-
-
136053-53-7, 125951-00-0
tert-butyl 4-methylcinnamate

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; palladium dichloride at 40℃; for 3h; Heck reaction; Inert atmosphere; neat (no solvent); | 100% |
| With triethylamine; palladium In N,N-dimethyl acetamide at 80℃; for 36h; Heck reaction; | 99% |
| With poly(N-isopropylacerylamide)-SCS-PdCl; TEA In n-heptane; N,N-dimethyl acetamide; water at 95℃; for 10h; Heck coupling; | 99% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
586-78-7
para-nitrophenyl bromide

-
-
370839-59-1
(E)-tert-butyl 3-(4-nitrophenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With tributyl-amine; [1,2-bis(4-Me-2-pyridylethynyl)benzene]dichloropalladium(II) at 100℃; for 7h; Heck reaction; | 100% |
| With {1,3-bis[2,6-bis(propan-2-yl)phenyl]-1,3-dihydro-2H-imidazol-2-ylidene}dichloro(pyridine)palladium; caesium carbonate In neat (no solvent) at 100℃; for 12h; Heck Reaction; | 99% |
| With C22H24Cl2N4Pd; tetrabutylammomium bromide; triethylamine In methanol at 70℃; for 24h; Reagent/catalyst; Concentration; Heck Reaction; | 97% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
696-62-8
para-iodoanisole

-
-
53484-52-9
tert-butyl (E)-3-(4-methoxyphenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate; palladium diacetate In N,N-dimethyl-formamide at 120℃; for 6h; Heck cross-coupling reaction; | 100% |
| With tetrabutyl ammonium fluoride; palladium dichloride at 60℃; for 12h; Heck reaction; Inert atmosphere; neat (no solvent); | 100% |
| With water; palladium diacetate; caesium carbonate at 140℃; for 4h; Heck reaction; Ionic liquid; Inert atmosphere; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
456-27-9
4-nitrobenzenediazonium tetrafluoroborate

-
-
370839-59-1
(E)-tert-butyl 3-(4-nitrophenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With 1,6,11-triazacyclopentadeca-3,8,12-triene Pd In ethanol at 20℃; for 2.75h; Heck reaction; | 100% |
| With 3-benzyl-1-(2-hydroxy-2-phenylethyl)imidazolium chloride; palladium diacetate In ethanol at 36℃; for 3h; Heck-Matsuda reaction; | 96% |
| With dichloro bis(acetonitrile) palladium(II) In water at 0 - 25℃; for 1.5h; | 96% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
52436-75-6
(4-tert-butylphenyl)diazonium tetrafluoroborate

| Conditions | Yield |
|---|---|
| With 1,6,11-triazacyclopentadeca-3,8,12-triene Pd In ethanol at 20℃; for 4h; Heck reaction; | 100% |
-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
1663-39-4
tert-Butyl acrylate

-
-
53484-52-9
tert-butyl (E)-3-(4-methoxyphenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With C31H38ClN3Pd; potassium carbonate In 1-methyl-pyrrolidin-2-one at 140℃; for 18h; Heck-Mizoroki coupling; Inert atmosphere; | 100% |
| With IMes-Pd(dmba)Cl; potassium carbonate In 1-methyl-pyrrolidin-2-one at 140℃; for 18h; Heck-Mizoroki reaction; Inert atmosphere; | 100% |
| With 1,3-disubstituted imidazolium bromide; potassium carbonate; palladium diacetate In 1,4-dioxane at 105℃; for 20h; Heck reaction; | 99% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
156-87-6
propan-1-ol-3-amine

-
-
644968-23-0
tert-butyl 3-[(2-tert-butoxycarbonyl-ethyl)-(3-hydroxy-propyl)-amino]-propionate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; Darkness; Inert atmosphere; | 100% |
| In methanol at 20 - 30℃; for 8h; | 99% |
| In methanol at 20℃; for 6h; | 98% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 10500, Mw/Mn = 1.18; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 10500, Mw/Mn = 1.18; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 1h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 20000, Mw/Mn = 1.12; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 20000, Mw/Mn = 1.12; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 1h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 29700, Mw/Mn = 1.09; monomer(s); tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 29700, Mw/Mn = 1.09; monomer(s); tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 2h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 47700, Mw/Mn = 1.06; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 47700, Mw/Mn = 1.06; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 2h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 58900, Mw/Mn = 1.05; monomer(s): tert-butyl acetate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 58900, Mw/Mn = 1.05; monomer(s): tert-butyl acetate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 2h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 83400, Mw/Mn = 1.05; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 83400, Mw/Mn = 1.05; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 2h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 250600, Mw/Mn = 1.17; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 250600, Mw/Mn = 1.17; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 3h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/Et3Al system, Mn = 170900, Mw/Mn = 1.06; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/Et3Al system, Mn = 170900, Mw/Mn = 1.06; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triethylaluminum In toluene at 0℃; for 1h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/Et3Al system, Mn = 305900, Mw/Mn = 1.25; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/Et3Al system, Mn = 305900, Mw/Mn = 1.25; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triethylaluminum In toluene at 0℃; for 2h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/Me3Al system, Mn = 437900, Mw/Mn = 1.61; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/Me3Al system, Mn = 437900, Mw/Mn = 1.61; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; trimethylaluminum In toluene at 0℃; for 2h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

- poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 5300, Mw/Mn = 1.26; monomer(s): tert-butyl acrylate
-
poly-tert-butyl acrylate, obtained by anionic polymerization, initiated with the tBuOK/iBu3Al system, Mn = 5300, Mw/Mn = 1.26; monomer(s): tert-butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In toluene at 0℃; for 1h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
146743-72-8
3-[Bis-(2-tert-butoxycarbonyl-ethyl)-amino]-propionic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 120℃; under 7500.75 Torr; for 3h; Microwave irradiation; | 100% |
| With ammonia In methanol at 20℃; for 6h; | 98% |
-
-
636-98-6
p-nitrobenzene iodide

-
-
1663-39-4
tert-Butyl acrylate

-
-
370839-59-1
(E)-tert-butyl 3-(4-nitrophenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium carbonate; palladium diacetate In N,N-dimethyl-formamide at 80℃; for 4h; Heck cross-coupling reaction; | 100% |
| With tetrabutyl ammonium fluoride; palladium dichloride at 40℃; for 3h; Heck reaction; Inert atmosphere; neat (no solvent); | 100% |
| With 1,4-diaza-bicyclo[2.2.2]octane; poly(ethylene glycol)-400; potassium carbonate; palladium diacetate In water at 80℃; for 4h; Heck cross-coupling; | 98% |
-
-
876061-13-1
2-(benzylsulfanyl)-N-(2-bromo-4,6-difluorophenyl)-N-methylacetamide

-
-
1663-39-4
tert-Butyl acrylate

-
-
876061-15-3
(E)-tert-butyl 3-(2-(2-(benzylsulfanyl)-N-methylacetamido)-3,5-difluorophenyl)acrylate

| Conditions | Yield |
|---|---|
| With triethylamine; tris-(o-tolyl)phosphine; palladium diacetate In o-xylene at 100℃; for 7h; Heck reaction; microwave irradiation; | 100% |
| With triethylamine; tris-(o-tolyl)phosphine; palladium diacetate In o-xylene at 100℃; for 7h; Heck coupling; microwave irradiation; | 87% |

| Conditions | Yield |
|---|---|
| With C33H33N2(1+)*Cl(1-); palladium diacetate; potassium carbonate In water; N,N-dimethyl-formamide for 2h; Reagent/catalyst; Heck Reaction; Inert atmosphere; Sealed tube; Heating; | 100% |
| With C30H42Cl2N2Pd; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 8h; Catalytic behavior; Reagent/catalyst; Heck Reaction; Inert atmosphere; Schlenk technique; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; Heck Reaction; | 98% |
-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 1h; | 100% |
-
-
1663-39-4
tert-Butyl acrylate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; triisobutylaluminum In hexane; toluene at 0℃; for 2h; | 100% |
tert-Butyl acrylate Consensus Reports
tert-Butyl acrylate Specification
The tert-Butyl acrylate, with the CAS registry number 1663-39-4, is also known as Acrylic acid, tert-butyl ester. It belongs to the product categories of Categories: Medical Intermediates; A-BAlphabetic; Alpha Sort; B; BI - BZAnalytical Standards; Chemical Class; Esters Gasoline, Diesel,& Petroleum; Olefins; Substance Classes; Volatiles/ Semivolatiles; Carbonyl Compounds; Acrylate Carbonyl Compounds; Acrylic Monomers; C6 to C7; Esters; Monomers. Its EINECS registry number is 216-768-7. This chemical's molecular formula is C7H12O2 and molecular weight is 128.17. Its IUPAC name is called tert-butyl prop-2-enoate.
Physical properties of tert-Butyl acrylate: (1)ACD/LogP: 2.02; (2)ACD/LogD (pH 5.5): 2.02; (3)ACD/LogD (pH 7.4): 2.02; (4)ACD/BCF (pH 5.5): 20.25; (5)ACD/BCF (pH 7.4): 20.25; (6)ACD/KOC (pH 5.5): 299.74; (7)ACD/KOC (pH 7.4): 299.74; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Index of Refraction: 1.417; (11)Molar Refractivity: 35.94 cm3; (12)Molar Volume: 142.7 cm3; (13)Surface Tension: 25.1 dyne/cm; (14)Density: 0.898 g/cm3; (15)Flash Point: 31.6 °C; (16)Enthalpy of Vaporization: 37.05 kJ/mol; (17)Boiling Point: 133 °C at 760 mmHg; (18)Vapour Pressure: 8.64 mmHg at 25°C.
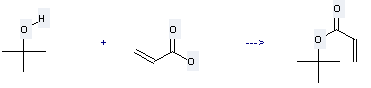
Preparation of tert-Butyl acrylate: this chemical can be prepared by acrylic acid and 2-methyl-propan-2-ol. This reaction will need reagents nickel (II)-acrylate and water.
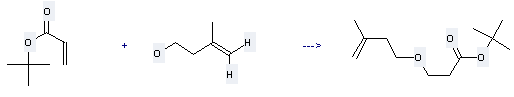
Uses of tert-Butyl acrylate: it can be used to produce 3-(3-methyl-but-3-enyloxy)-propionic acid tert-butyl ester at temperature of 50 °C. This reaction will need reagent Triton B with reaction time of 2.5 hours. The yield is about 91%.
This chemical has so many characteristics: hydrolysis stabilizers, light inert, high Tg, globular polymer chain structure, hydrophilic and so on. In addition, it can be used in coating surface varnish, vehicle repair light, high polymer paper conditioner, nursing polymer products, carbonyl reforming polyolefin. Meanwhile, tert-Butyl acrylate is also an important raw material in organic synthetic.
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and harmful. It is harmful by inhalation, in contact with skin and if swallowed. In addition, it is irritating to respiratory system and skin. You should keep it away from sources of ignition - No smoking. What's more, you must avoid contact with eyes. Whenever you will contact it, please wear suitable gloves.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(C)(C)OC(=O)C=C
(2)InChI: InChI=1S/C7H12O2/c1-5-6(8)9-7(2,3)4/h5H,1H2,2-4H3
(3)InChIKey: ISXSCDLOGDJUNJ-UHFFFAOYSA-N
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate
- tert-Butyl (2R)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- tert-Butyl (2S)-2-carbamoyl-2,3-dihydropyrrole-1-carboxylate
- tert-Butyl (3S)-3-(hydroxymethyl)morpholine-4-carboxylate
- tert-Butyl (3S)-3-amino-4-phenylbutanoate
- tert-Butyl (3S,4R)-4-(2-methoxyphenyl)pyrrolidin-3-ylcarbamate
- tert-Butyl (3S,4R)-4-phenylpyrrolidin-3-ylcarbamate
- tert-Butyl (4-aminophenyl)carbamate
- 1663-45-2
- 16634-82-5
- 16634-88-1
- 16634-90-5
- 16634-91-6
- 16635-95-3
- 1663-61-2
- 16636-62-7
- 1663-67-8
- 166386-48-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View