Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Zhuozhou Wenxi import and Export Co., Ltd
Product Description Description & Specification Category Pharmaceutical Raw Materials, Fine Chemicals, Bulk drug Standard Medical standard
Cas:13465-08-2
Min.Order:1 Kilogram
FOB Price: $112.0
Type:Trading Company
inquirySparrow Chemical Co.,Ltd
Our company provides the best service for our customers. Under the premise of ensuring quality and delivery date, give customers the best price. The company is more willing to meet our customers. If you want to visit our factories and warehouses, we
NovaChemistry
high purity Application:Drug intermediates Materials intermediates and active molecules
Synthetic route

| Conditions | Yield |
|---|---|
| In neat (no solvent) reaction by influence of O3 on undiluted NH2OH;; condensation by cooling in a ether CO2 mixture;; |

| Conditions | Yield |
|---|---|
| In not given evapn. of soln. in vac. at lower temp., residue with alcohol absorpted; | |
| In not given evapn. of soln. in vac. at lower temp., residue with alcohol absorpted; |
-
-
3811-04-9
potassium chlorate

-
-
15588-62-2
hydroxylammonium perchlorate

-
-
13465-08-2
hydroxylamine nitrate

| Conditions | Yield |
|---|---|
| In water mixing components solns. (small amount of NH3OH-salt), water removal (distn.) to dryness; no isolation in pure state; IR spectroscopy; |

| Conditions | Yield |
|---|---|
| With ozone evapn. of soln. over H2SO4; | |
| With ozone evapn. of soln. over H2SO4; |
-
-
66857-72-5
hydroxylammonium chlorate

-
A
-
13465-08-2
hydroxylamine nitrate

-
B
-
10049-04-4, 25052-55-5
chlorine dioxide

| Conditions | Yield |
|---|---|
| In water decompn. in aq. soln.; |
-
-
7616-94-6
perchloryl fluoride

-
-
7803-49-8
hydroxylamine

-
A
-
13465-08-2
hydroxylamine nitrate

-
B
-
17256-78-9
hydroxylammonium fluoride

| Conditions | Yield |
|---|---|
| In ethanol -70 to -60°C, storing (120 h), gradual increase of temperature; IR spectroscopy; |

-
-
7803-49-8
hydroxylamine

-
-
13465-08-2
hydroxylamine nitrate

| Conditions | Yield |
|---|---|
| With ozone ice cooling in an ether-CO2 mixture; | |
| With ozone ice cooling in an ether-CO2 mixture; |

| Conditions | Yield |
|---|---|
| In water byproducts: BaSO4; conc. soln. of (NH2OH)2*H2SO4 added to Ba(NO3)2 soln. heated to 60 °C; BaSO4 sepd, by centrifugation; |
-
-
66857-72-5
hydroxylammonium chlorate

-
-
13465-08-2
hydroxylamine nitrate

| Conditions | Yield |
|---|---|
| slow dehydration of small amt. of comp. to avoid expolosion; |
-
-
100-64-1
Cyclohexanone oxime

-
-
13465-08-2
hydroxylamine nitrate

| Conditions | Yield |
|---|---|
| With nitric acid In ethylbenzene at 40℃; under 1500.15 Torr; for 0.666667h; |

| Conditions | Yield |
|---|---|
| With europium(III) nitrate hexahydrate; water; nitric acid pH=Ca. 0.28 - Ca. 1.21; Kinetics; |
-
-
13465-08-2
hydroxylamine nitrate

-
A
-
7727-37-9
nitrogen

-
B
-
7732-18-5
water

-
C
-
80937-33-3
oxygen

-
D
-
10102-44-0
Nitrogen dioxide

| Conditions | Yield |
|---|---|
| With catalyst: Pt/Si-doped Al2O3 In water catalytic decompn. of NH3OH(NO3)/H2O energetic liquid at 45°C on Pt supported on Si-doped alumina; |

-
-
13465-08-2
hydroxylamine nitrate

-
A
-
7727-37-9
nitrogen

-
B
-
7732-18-5
water

-
C
-
10102-43-9
nitrogen(II) oxide

| Conditions | Yield |
|---|---|
| With catalyst: Pt/(Al2O3)0.88(SiO2)012 In water byproducts: NO2, N2O, O2; 79% aq. NH3OHNO3 decompd. in increasing temp. mode or at 40°C in const. volume batch reactor; monitored by online analysis by mass spectrometer; |

-
-
13465-08-2
hydroxylamine nitrate

-
A
-
7732-18-5
water

-
B
-
7697-37-2
nitric acid

-
C
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; 463 - 523 K, 27.5 MPa; |

-
-
13465-08-2
hydroxylamine nitrate

-
A
-
7732-18-5
water

-
B
-
10102-43-9
nitrogen(II) oxide

-
C
-
10102-44-0
Nitrogen dioxide

-
D
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; between 84.8-120.9°C; |

-
-
13465-08-2
hydroxylamine nitrate

-
-
7697-37-2
nitric acid

| Conditions | Yield |
|---|---|
| In water Kinetics; byproducts: H2O, N2O, N2; aq. soln. of nitrate was heated at 2°C/min; |
-
-
13465-08-2
hydroxylamine nitrate

-
A
-
7697-37-2
nitric acid

-
B
-
10102-44-0
Nitrogen dioxide

-
C
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| In solid Ar atmosphere; thermolysis of solid hydroxylammonium nitrate (15-500 psi Ar, heating rate 130 K/s); | |
| With cobalt doped cerium oxide at 120 - 130℃; Thermodynamic data; Reagent/catalyst; Inert atmosphere; |

-
-
13465-08-2
hydroxylamine nitrate

-
-
10102-44-0
Nitrogen dioxide

| Conditions | Yield |
|---|---|
| In water decompn. above 110°C; | |
| In water decompn. above 110°C; |

| Conditions | Yield |
|---|---|
| In not given Kinetics; byproducts: water; | |
| In not given Kinetics; byproducts: water; |

| Conditions | Yield |
|---|---|
| In ethanol; water -15°C; |

| Conditions | Yield |
|---|---|
| In not given Kinetics; byproducts: water; | |
| In not given Kinetics; byproducts: water; |

| Conditions | Yield |
|---|---|
| In not given Kinetics; byproducts: water; | |
| In not given Kinetics; byproducts: water; |

| Conditions | Yield |
|---|---|
| In not given Kinetics; byproducts: water; | |
| In not given Kinetics; byproducts: water; |

| Conditions | Yield |
|---|---|
| In not given Kinetics; byproducts: water; | |
| In not given Kinetics; byproducts: water; |

| Conditions | Yield |
|---|---|
| In not given Kinetics; byproducts: water; | |
| In not given Kinetics; byproducts: water; |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View

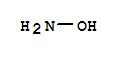




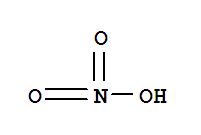
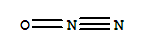
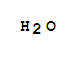
 Xn;
Xn;  Xi;
Xi;  C
C