Hunan Wistar Imp. & Exp. Co., Ltd.
The company serves as a key global supplier of statins intermediates, which has a solid industrial foundation in the field of statins for lipid-lowering drugs, and holds a leading position in the market. Leveraging extensive experience in research an
Cas:147118-36-3
Min.Order:25 Kilogram
Negotiable
Type:Trading Company
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:147118-36-3
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquirySinoway Industrial Co., Ltd.
Assay: 99% up; Stable supply with over 50Mt annually; Appearance:white to off-white powder Storage:room temprature Package:25kg/drum Application:Intermediate of Rosuvastatin Calcium Port:Beijing/Shanghai/Shenzhen/Hangzhou
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $75.0 / 85.0
Type:Trading Company
inquiryWuhan Fortuna Chemical Co.,Ltd
Unique advantages Guaranteed purity High quality & competitive price Quality control Fast feedback Prompt shipment Appearance:White to off-white crystalline powder Storage:cool dry place Package:25kg/drum Application:pharmaceutical interme
Cas:147118-36-3
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryHenan Allgreen Chemical Co.,Ltd
Good quality Good price Promptly delivery Appearance:white powder Storage:dry dondition Package:According to the demand of customer Application:intermediate Transportation:According to the demand of customer Port:shanghai
Cas:147118-36-3
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:147118-36-3
Min.Order:1
Negotiable
Type:Other
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:147118-36-3
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryShanghai SE Pharm Co., Ltd
1. made in GMP plant, commerially 2. Normal Stock: 500kgs 3. Audit accepted. Related documents are available to offer and audited by many clients, such as Lupin, MSN, Dr reddy etc 4. Chromatographic Purity (HPLC): not less than 99.0% Appearan
Cas:147118-36-3
Min.Order:1 Gram
Negotiable
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem’s R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:147118-36-3
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryGIHI CHEMICALS CO.,LIMITED
GIHI CHEMICALS exported this product to many countries and regions at best price. if you are looking for the material's manufacturer or supplier in china, GIHI CHEMICALS is your best choice. pls contact with us freely for getting detailed prod
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryJinan Finer Chemical Co., Ltd
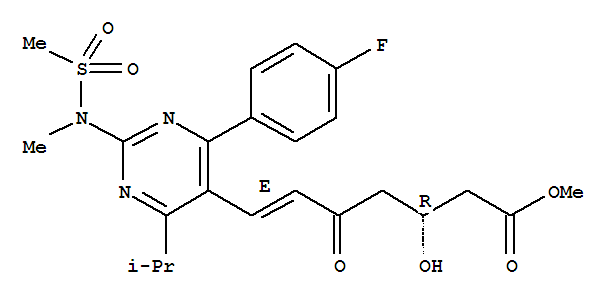
Product Description Product website: http://www.finerchem.com/pro01en/id/1032.html Product Name 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-me
Cas:147118-36-3
Min.Order:1 Gram
FOB Price: $45.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-methanol Synonyms: 4-(4-FLUOROPHENYL)-6-ISOPROPYL-2-(N-METHYL-N-METHYLSULFONYLAMINO)PYRIMIDINE-5-METHANOL;4-(4-FLUOROPHENYL)-6-ISOPROPYL-2-[(N-M
Cas:147118-36-3
Min.Order:1 Gram
FOB Price: $8900.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:147118-36-3
Min.Order:10 Kilogram
FOB Price: $80.0 / 100.0
Type:Trading Company
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by email in time prod
Cas:147118-36-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Sinotech Import&Export Corporation
1. Characteristics Item Specification CAS 147118-36-3 Appearance White powder 2. Application: Pharmaceutical Intermediates. Appearance: white powder Storage:Store in
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Other
inquiryHubei Langyou International Trading Co., Ltd
1. Guaranteed purity; 2. Large quantity in stock; 3. Largest manufacturer; 4. Best service after shipment with email; 5. High quality & competitive price; Appearance:White Crystalline Powder Storage:Store in sealed conta
Cas:147118-36-3
Min.Order:10 Gram
Negotiable
Type:Other
inquiryHANWAYS CHEMPHARM CO.,LIMITED
Hanways chempharm is a specialized company concentrating on the R&D, production, marketing and technical service of APIs and pharmaceutical intermediates. The marketing department is located in Wuhan. We have two GMP facilities in Hubei Pr
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $260.0 / 300.0
Type:Trading Company
inquiryShanghai Minstar Chemical Co., Ltd
Product Name: 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-methanol CAS: 147118-36-3 MF: C16H20FN3O3S MW: 353.41 EINECS: 604-563-9 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)ami
Cas:147118-36-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:147118-36-3
Min.Order:1 Kilogram
FOB Price: $80.0 / 100.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-methanol CAS:147118-36-3 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and tr
Cas:147118-36-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryWuhan Zenuo Biological Medicine Technology Co Ltd
Product Name: 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-methanol Synonyms: 4-(4-FLUOROPHENYL)-6-ISOPROPYL-2-(N-METHYL-N-METHYLSULFONYLAMINO)PYRIMIDINE-5-METHANOL;4-(4-FLUOROPHENYL)-6-ISOPROPYL-2-[(N-METHYL
Cas:147118-36-3
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:147118-36-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryXiamen Hisunny Chemical Co.,Ltd
Best quality & Attractive price & Professional service; Trial & Pilot & Commercial Hisunny Chemical is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality intermediates, specia
Cas:147118-36-3
Min.Order:0
Negotiable
Type:Manufacturers
inquiryTriumph International Development Limilted
Appearance:white powder Storage:Store in a cool,dry place and keep away from direct strong light Package:As customer request Application:Used for research and industrial manufacture. Transportation:Common products:Sea/Air/Courie
Cas:147118-36-3
Min.Order:100 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:147118-36-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryNanjing Fred Technology Co.,Ltd.
4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-methanol is one of the most competitive products in our company, we can supply it with good quality and best price. Appearance:White to light yellow to light orange so
Cas:147118-36-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:147118-36-3
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquirySynthetic route
-
-
289042-11-1
methyl 4-(4-fluorophenyl)-6-isopropyl-2-(N-methanesulphonyl-N-methylamino)pyrimidine-5-carboxylate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: methyl 4-(4-fluorophenyl)-6-isopropyl-2-(N-methanesulphonyl-N-methylamino)pyrimidine-5-carboxylate With diisobutylaluminium hydride In hexane; toluene at -10℃; for 1h; Stage #2: With hydrogenchloride In methanol; hexane; water; toluene at -10 - 40℃; for 0.333333h; | 100% |
| Stage #1: methyl 4-(4-fluorophenyl)-6-isopropyl-2-(N-methanesulphonyl-N-methylamino)pyrimidine-5-carboxylate With sodium tetrahydroborate; boron trifluoride diethyl etherate In tetrahydrofuran at 0 - 60℃; for 20h; Stage #2: With hydrogenchloride In tetrahydrofuran; water for 1.5h; Temperature; Reagent/catalyst; Solvent; | 96.7% |
| Stage #1: methyl 4-(4-fluorophenyl)-6-isopropyl-2-(N-methanesulphonyl-N-methylamino)pyrimidine-5-carboxylate With diisobutylaluminium hydride In toluene at -15 - -5℃; for 2 - 2.5h; Stage #2: With hydrogenchloride; water In toluene at -10 - 40℃; for 1 - 1.33333h; Stage #3: With sodium hydrogencarbonate In water; ethyl acetate Product distribution / selectivity; | 93% |
-
-
799842-07-2
N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran for 6h; Reflux; | 100% |
| With oxygen; eosin y In dimethyl sulfoxide at 40℃; Irradiation; | 82% |
| With water; sodium hydrogencarbonate In acetonitrile for 4h; Reflux; | |
| With water; sodium hydrogencarbonate In acetonitrile for 4h; Reflux; | |
| With sodium hydrogencarbonate In acetonitrile for 4h; Reflux; |
-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 5 - 25℃; for 2h; | 98% |
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 5 - 10℃; | |
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 0 - 20℃; for 1h; |
-
-
1263475-93-9
4-(4-fluorophenyl)-6-isopropyl-2-(methanesulfonyl(methyl)amino)pyrimidine-5-carboxylic acid

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With lithium borohydride; boron trifluoride diethyl etherate In tetrahydrofuran at -5 - 20℃; for 4h; Reagent/catalyst; Temperature; | 97.8% |
| With chloro-trimethyl-silane; potassium borohydride In tetrahydrofuran at 65℃; for 30h; Temperature; Reagent/catalyst; Inert atmosphere; | 97% |
| Stage #1: 4-(4-fluorophenyl)-6-isopropyl-2-(methanesulfonyl(methyl)amino)pyrimidine-5-carboxylic acid With potassium borohydride In tetrahydrofuran for 0.0833333h; Cooling with ice; Stage #2: With aluminum (III) chloride In tetrahydrofuran at 74℃; for 4.08333h; Reagent/catalyst; | 97.9% |
| With sodium tetrahydroborate; boron trifluoride diethyl etherate In tetrahydrofuran at -5 - 30℃; Reagent/catalyst; Temperature; | 96.4% |
| With sodium tetrahydroborate; chloro-trimethyl-silane In tetrahydrofuran for 24h; Reagent/catalyst; Reflux; Inert atmosphere; | 92% |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol for 4h; | 88.1% |
-
-
147118-30-7
ethyl 2-(N-methyl-N-methanesulfonylamino)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-carboxylic acid

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(N-methyl-N-methanesulfonylamino)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-carboxylic acid With diisobutylaluminium hydride In toluene at -78 - 0℃; Inert atmosphere; Stage #2: With water In toluene | 81% |
| With diisobutylaluminium hydride In toluene at -74℃; for 1h; | 277 mg |
| Multi-step reaction with 2 steps 1: lithium hydroxide monohydrate / water; methanol / 60 - 70 °C 2: lithium borohydride; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / -5 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: lithium hydroxide monohydrate; water / methanol / 60 - 70 °C 2: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / -5 - 30 °C View Scheme |
-
-
459-57-4
4-fluorobenzaldehyde

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 86.7 percent / piperidine, AcOH / benzene / Heating 2: 1.) HMPA, 2.) DDQ / 1.) 100 deg C, 22 h, 2.) benzene, 30 min 3: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 4: 46.9 g / ethanol / 1 h / Ambient temperature 5: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 6: 277 mg / DIBAL-H / toluene / 1 h / -74 °C View Scheme |
-
-
122930-45-4
(E)-2-<(4-fluorophenyl)methylene>-4-methyl-3-oxopentanoic acid ethyl ester

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1.) HMPA, 2.) DDQ / 1.) 100 deg C, 22 h, 2.) benzene, 30 min 2: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 3: 46.9 g / ethanol / 1 h / Ambient temperature 4: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 5: 277 mg / DIBAL-H / toluene / 1 h / -74 °C View Scheme |
-
-
7152-15-0
ethyl 4-methyl-3-oxo-pentanoate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 86.7 percent / piperidine, AcOH / benzene / Heating 2: 1.) HMPA, 2.) DDQ / 1.) 100 deg C, 22 h, 2.) benzene, 30 min 3: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 4: 46.9 g / ethanol / 1 h / Ambient temperature 5: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 6: 277 mg / DIBAL-H / toluene / 1 h / -74 °C View Scheme |
-
-
147118-32-9
5-ethoxycarbonyl-6-(4'-fluorophenyl)-4-isopropyl-2-(methylamino)pyrimidine

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 2: 277 mg / DIBAL-H / toluene / 1 h / -74 °C View Scheme |
-
-
147118-27-2
ethyl 4-(4-fluorophenyl)-2-(methylsulfanyl)-6-(propan-2-yl)-pyrimidine-5-carboxylate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 2: 46.9 g / ethanol / 1 h / Ambient temperature 3: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 4: 277 mg / DIBAL-H / toluene / 1 h / -74 °C View Scheme |
-
-
147118-28-3
ethyl 4-(4-fluorophenyl)-6-isopropyl-2-(methylsulfonyl)pyrimidine-5-carboxylate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 46.9 g / ethanol / 1 h / Ambient temperature 2: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 3: 277 mg / DIBAL-H / toluene / 1 h / -74 °C View Scheme |
-
-
953776-62-0
N-(4-(4-fluorophenyl)-6-isopropyl-5-methylpyrimidin-2-yl)-N-methylmethanesulfonamide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 2: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux View Scheme | |
| Stage #1: N-(4-(4-fluorophenyl)-6-isopropyl-5-methylpyrimidin-2-yl)-N-methylmethanesulfonamide With N-Bromosuccinimide In acetonitrile at 20℃; for 4h; UV-irradiation; Stage #2: With sodium hydrogencarbonate In acetonitrile for 4h; Reflux; Stage #3: With water In acetonitrile at 20℃; | |
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 2: water / tetrahydrofuran / 6 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / acetonitrile / 68 h / 20 °C / UV-irradiation 2: sodium hydrogencarbonate / acetonitrile / 4 h / Reflux View Scheme |
-
-
114433-94-2
1-(4-Fluorophenyl)-3-isopropylpropan-1,3-dione

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 24 h / 20 °C 2: caesium carbonate / 2-methyltetrahydrofuran / 10 h / 40 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 5: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 48 h / 20 °C / Inert atmosphere 2: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 5: water / tetrahydrofuran / 6 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 48 h / 20 °C / Inert atmosphere 2: caesium carbonate / 2-methyltetrahydrofuran / 24 h / 70 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 5: water / tetrahydrofuran / 6 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1.1: potassium carbonate / acetone / 24 h / 20 °C 2.1: tetrahydrofuran / 0.17 h / 20 °C 2.2: 8 h / 40 °C 3.1: triethylamine / dichloromethane / 26 h / 0 - 20 °C 4.1: N-Bromosuccinimide / acetonitrile / 68 h / 20 °C / UV-irradiation 5.1: sodium hydrogencarbonate / acetonitrile / 4 h / Reflux View Scheme |
-
-
1207460-34-1
4-(4-fluorophenyl)-6-isopropyl-N,5-dimethylpyrimidin-2-amine

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 3: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 3: water / tetrahydrofuran / 6 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 26 h / 0 - 20 °C 2: N-Bromosuccinimide / acetonitrile / 68 h / 20 °C / UV-irradiation 3: sodium hydrogencarbonate / acetonitrile / 4 h / Reflux View Scheme |
-
-
1354455-77-8
1-(4-fluorophenyl)-2-methyl-3-isopropylpropan-1,3-dione

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: caesium carbonate / 2-methyltetrahydrofuran / 10 h / 40 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 4: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: water / tetrahydrofuran / 6 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: caesium carbonate / 2-methyltetrahydrofuran / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: water / tetrahydrofuran / 6 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: tetrahydrofuran / 0.17 h / 20 °C 1.2: 8 h / 40 °C 2.1: triethylamine / dichloromethane / 26 h / 0 - 20 °C 3.1: N-Bromosuccinimide / acetonitrile / 68 h / 20 °C / UV-irradiation 4.1: sodium hydrogencarbonate / acetonitrile / 4 h / Reflux View Scheme |
-
-
953776-62-0
N-(4-(4-fluorophenyl)-6-isopropyl-5-methylpyrimidin-2-yl)-N-methylmethanesulfonamide

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 2: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
799842-07-2
N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide at 20℃; for 71h; Kornblum oxidation; Inert atmosphere; |
-
-
1354455-77-8
1-(4-fluorophenyl)-2-methyl-3-isopropylpropan-1,3-dione

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: caesium carbonate / 2-methyltetrahydrofuran / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
1207460-34-1
4-(4-fluorophenyl)-6-isopropyl-N,5-dimethylpyrimidin-2-amine

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 3: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
114433-94-2
1-(4-Fluorophenyl)-3-isopropylpropan-1,3-dione

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 48 h / 20 °C / Inert atmosphere 2: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 5: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 48 h / 20 °C / Inert atmosphere 2: caesium carbonate / 2-methyltetrahydrofuran / 24 h / 70 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 5: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
94250-56-3
4-methyl-3-oxopentanoic acid benzyl ester

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 12 h / 50 °C 2.1: sodium nitrite; nitric acid / dichloromethane / 1 h / 0 °C 3.1: potassium carbonate; acetic acid butyl ester; p-toluenesulfonyl chloride / 2 h / 40 °C 3.2: 12 h / 120 °C 4.1: 5%-palladium/activated carbon; hydrogen / methanol / 5 h / 20 °C 5.1: sodium tetrahydroborate; chloro-trimethyl-silane / tetrahydrofuran / 24 h / Reflux; Inert atmosphere View Scheme |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium nitrite; nitric acid / dichloromethane / 1 h / 0 °C 2.1: potassium carbonate; acetic acid butyl ester; p-toluenesulfonyl chloride / 2 h / 40 °C 2.2: 12 h / 120 °C 3.1: 5%-palladium/activated carbon; hydrogen / methanol / 5 h / 20 °C 4.1: sodium tetrahydroborate; chloro-trimethyl-silane / tetrahydrofuran / 24 h / Reflux; Inert atmosphere View Scheme |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; acetic acid butyl ester; p-toluenesulfonyl chloride / 2 h / 40 °C 1.2: 12 h / 120 °C 2.1: 5%-palladium/activated carbon; hydrogen / methanol / 5 h / 20 °C 3.1: sodium tetrahydroborate; chloro-trimethyl-silane / tetrahydrofuran / 24 h / Reflux; Inert atmosphere View Scheme |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 5%-palladium/activated carbon; hydrogen / methanol / 5 h / 20 °C 2: sodium tetrahydroborate; chloro-trimethyl-silane / tetrahydrofuran / 24 h / Reflux; Inert atmosphere View Scheme |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 150 °C 2.1: copper(l) chloride; sulfuric acid / methanol / 12 h / 50 °C 3.1: sodium nitrite; nitric acid / dichloromethane / 1 h / 0 °C 4.1: potassium carbonate; acetic acid butyl ester; p-toluenesulfonyl chloride / 2 h / 40 °C 4.2: 12 h / 120 °C 5.1: 5%-palladium/activated carbon; hydrogen / methanol / 5 h / 20 °C 6.1: sodium tetrahydroborate; chloro-trimethyl-silane / tetrahydrofuran / 24 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 24 h / 80 °C / Inert atmosphere 2.1: copper dichloride; potassium carbonate; tert.-butylhydroperoxide / dichloromethane / 7 h / 40 °C 3.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 4.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 4.2: 7 h / Reflux 5.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 24 h / 80 °C / Inert atmosphere 2.1: copper dichloride; potassium carbonate; tert.-butylhydroperoxide / dichloromethane / 7 h / 40 °C 3.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 4.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 4.2: 7 h / Reflux 5.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 9 h / 78 °C 2.1: dipotassium peroxodisulfate / water / Reflux 3.1: potassium carbonate; p-toluenesulfonyl chloride / acetic acid butyl ester / 0.5 h / 45 °C 3.2: 3 h / 125 °C 4.1: sodium hydroxide / ethanol / 0.5 h / 82 °C 5.1: potassium borohydride / tetrahydrofuran / 0.08 h / Cooling with ice 5.2: 4.08 h / 74 °C View Scheme |
-
-
459-57-4
4-fluorobenzaldehyde

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 24 h / 80 °C / Inert atmosphere 2.1: copper dichloride; potassium carbonate; tert.-butylhydroperoxide / dichloromethane / 7 h / 40 °C 3.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 4.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 4.2: 7 h / Reflux 5.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 24 h / 80 °C / Inert atmosphere 2.1: copper dichloride; potassium carbonate; tert.-butylhydroperoxide / dichloromethane / 7 h / 40 °C 3.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 4.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 4.2: 7 h / Reflux 5.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(l) chloride; sulfuric acid / methanol / 9 h / 78 °C 2.1: dipotassium peroxodisulfate / water / Reflux 3.1: potassium carbonate; p-toluenesulfonyl chloride / acetic acid butyl ester / 0.5 h / 45 °C 3.2: 3 h / 125 °C 4.1: sodium hydroxide / ethanol / 0.5 h / 82 °C 5.1: potassium borohydride / tetrahydrofuran / 0.08 h / Cooling with ice 5.2: 4.08 h / 74 °C View Scheme |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: copper dichloride; potassium carbonate; tert.-butylhydroperoxide / dichloromethane / 7 h / 40 °C 2.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 3.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 3.2: 7 h / Reflux 4.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: copper dichloride; potassium carbonate; tert.-butylhydroperoxide / dichloromethane / 7 h / 40 °C 2.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 3.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 3.2: 7 h / Reflux 4.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: dipotassium peroxodisulfate / water / Reflux 2.1: potassium carbonate; p-toluenesulfonyl chloride / acetic acid butyl ester / 0.5 h / 45 °C 2.2: 3 h / 125 °C 3.1: sodium hydroxide / ethanol / 0.5 h / 82 °C 4.1: potassium borohydride / tetrahydrofuran / 0.08 h / Cooling with ice 4.2: 4.08 h / 74 °C View Scheme |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 2.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 2.2: 7 h / Reflux 3.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: trichlorophosphate; N,N-dimethyl-aniline / 2.5 h / 80 °C / Inert atmosphere 2.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 2.2: 7 h / Reflux 3.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium carbonate; p-toluenesulfonyl chloride / acetic acid butyl ester / 0.5 h / 45 °C 1.2: 3 h / 125 °C 2.1: sodium hydroxide / ethanol / 0.5 h / 82 °C 3.1: potassium borohydride / tetrahydrofuran / 0.08 h / Cooling with ice 3.2: 4.08 h / 74 °C View Scheme |
-
-
488798-38-5
2-chloro-4-(4-fluoro-phenyl)-6-isopropyl-pyrimidine-5-carboxylic acid methyl ester

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydride / mineral oil; acetonitrile / 0.08 h 1.2: 7 h / Reflux 2.1: diisobutylaluminium hydride / toluene / 1.5 h / -15 - 0 °C View Scheme |
-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 9-azabicyclo<3.3.1>nonane-N-oxyl In acetonitrile at 20 - 50℃; for 2h; Sealed tube; | 99% |
| With fluorosulfonyl fluoride; potassium carbonate; dimethyl sulfoxide at 20℃; for 2h; Sealed tube; chemoselective reaction; | 99% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper(II) nitrate trihydrate In 2-methyltetrahydrofuran at 40℃; for 18h; Temperature; Reagent/catalyst; Solvent; | 99% |
-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
799842-07-2
N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With phosphorus tribromide In toluene; acetonitrile at 10 - 20℃; for 1h; Inert atmosphere; | 99% |
| With phosphorus tribromide In dichloromethane at 0 - 10℃; for 1h; | 90.6% |
| With phosphorus tribromide In dichloromethane at 20℃; for 1h; | 82% |
-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; caesium carbonate at 20℃; for 12h; Sealed tube; | 99% |
-
-
66003-78-9
triphenylsulfonium trifluoromethanesulfonate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With cesium hydroxide In 1,4-dioxane at 50℃; for 24h; Inert atmosphere; Glovebox; | 99% |
-
-
603-35-0
triphenylphosphine

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In acetonitrile Solvent; Reflux; | 98% |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| In acetonitrile for 24h; Reflux; | 97% |
-
-
191419-22-4
ethyl 5-(bromomethyl)-1,3-diphenyl-1H-pyrazole-4-carboxylate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium hydroxide In tetrahydrofuran at 20℃; | 95% |
-
-
6399-81-1
triphenylphosphine hydrobromide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| In toluene for 10h; Reflux; | 94% |
| In toluene for 10h; Reflux; | 83% |
-
-
811-68-7
silver(I) trifluoromethanethiolate

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide In toluene at 80℃; for 10h; Schlenk technique; Inert atmosphere; | 93% |
-
-
998-40-3
tributylphosphine

-
-
407-25-0
trifluoroacetic anhydride

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: trifluoroacetic anhydride; N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide With triethylamine In acetic acid butyl ester at 20 - 60℃; Stage #2: tributylphosphine In acetic acid butyl ester for 2h; Product distribution / selectivity; Heating / reflux; | 90% |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
925422-06-6
N-[5-chloromethyl-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-2-yl]-N-methyl-methanesulfonamide

| Conditions | Yield |
|---|---|
| With methanesulfonyl chloride; triethylamine In dichloromethane at 0 - 25℃; for 5h; | 88% |

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With 1,2-Diiodoethane; tetra-(n-butyl)ammonium iodide; triphenylphosphine In N,N-dimethyl-formamide; acetonitrile at 80℃; for 0.25h; Inert atmosphere; Sealed tube; | 87% |
-
-
75264-92-5
tetramethylammonium trifluoromethylselenate(0)

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With calcium chloride In acetonitrile at 80℃; for 2h; Time; Inert atmosphere; Sealed tube; Glovebox; | 87% |
-
-
4020-99-9
diphenylphosphinous acid methyl ester

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
289042-10-0
N-(5-((diphenylphosphoryl)methyl)-4-(4-fluorophenyl)- 6-isopropylpyrimidin-2-yl)-N-methylmethansulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide With phosphorus tribromide In acetonitrile at 20℃; Stage #2: diphenylphosphinous acid methyl ester at 60℃; | 86% |
-
-
799842-07-2
N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene for 12h; Reflux; | 82.4% |
-
-
139-02-6
sodium phenoxide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide With 1,2-Diiodoethane; N,N-dimethyl-formamide; triphenylphosphine at 20℃; for 0.0166667h; Sealed tube; Inert atmosphere; Stage #2: sodium phenoxide at 20℃; Sealed tube; Inert atmosphere; | 77% |
-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium fluoride; 2-fluoropyridine; silver trifluoromethanesulfonate; Selectfluor In ethyl acetate at 20℃; for 12h; Glovebox; Inert atmosphere; | 76% |
-
-
124898-13-1
trimethyl(pentafluoroethyl)silane

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium fluoride; 2-fluoropyridine; silver trifluoromethanesulfonate; lithium trifluoromethanesulfonate; Selectfluor In ethyl acetate at 20℃; for 12h; Inert atmosphere; | 64% |
-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium 2-(difluoro(trifluoromethoxy)methoxy)-2,2-difluoroacetate; tetramethylammonium fluoride at 150℃; for 5h; Glovebox; Inert atmosphere; Schlenk technique; Sealed tube; | 57% |
-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With acetone; lithium diisopropyl amide In tetrahydrofuran at -10℃; for 4.5h; Temperature; | 49.6% |
-
-
67-64-1
acetone

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -10℃; for 4.5h; Temperature; | 49.6% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View