Hunan Wistar Imp. & Exp. Co., Ltd.
The company serves as a key global supplier of statins intermediates, which has a solid industrial foundation in the field of statins for lipid-lowering drugs, and holds a leading position in the market. Leveraging extensive experience in research an
Cas:147118-37-4
Min.Order:25 Kilogram
Negotiable
Type:Trading Company
inquiryDayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product spe
Cas:147118-37-4
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:147118-37-4
Min.Order:1 Metric Ton
Negotiable
Type:Manufacturers
inquirySinoway Industrial Co., Ltd.
Assay: 99% up; Stable supply with over 50Mt annually; Appearance:white to off-white powder Storage:room temprature Package:25kg/drum Application:Intermediate of Rosuvastatin Calcium Port:Beijing/Shanghai/Shenzhen/Hangzhou
Cas:147118-37-4
Min.Order:1 Kilogram
FOB Price: $75.0 / 85.0
Type:Trading Company
inquiryHangzhou Think Chemical Co. Ltd
4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl CAS No.:147118-37-4 Name: 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl Synon
Cas:147118-37-4
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Other
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Appearance:white powder Storage:Sealed, dry, microtherm , avoid light and smell Package:According to the demand of customer Application:Pharmaceutical intermediates Transportation:by air or by sea Port:shanghai
Cas:147118-37-4
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:147118-37-4
Min.Order:1
Negotiable
Type:Other
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:147118-37-4
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:147118-37-4
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHebei yanxi chemical co.,LTD.
1 Factory price 2 High quality 3 Good service 4 Prompt service Triclosan, scientific name "two chlorobenzene oxygen chlorophenol", the chemical formula for C12H7Cl3O2, also known as its "new", "triclosan", etc.,
Cas:147118-37-4
Min.Order:500 Kilogram
FOB Price: $9000.0 / 10000.0
Type:Trading Company
inquiryJinan Finer Chemical Co., Ltd
Product Description Product website: http://www.finerchem.com/pro01en/id/1033.html Product Name 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-f
Cas:147118-37-4
Min.Order:1 Gram
FOB Price: $45.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl Synonyms: Methyl[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-sulfonyl-amino)-pyrimidin-5-yl]carboxylate;4-(4-Fluorophenyl)-5-brom
Cas:147118-37-4
Min.Order:1 Gram
FOB Price: $8900.0
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:147118-37-4
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by email in time prod
Cas:147118-37-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHubei Langyou International Trading Co., Ltd
Advantages: Hubei XinRunde Chemical Co., Ltd is a renowned pharmaceutical manufacturer. We can offer high quality products at competitive price in quick delivery with 100% custom pass guaranteed. Never stop striving to offer our best s
Cas:147118-37-4
Min.Order:10 Gram
Negotiable
Type:Other
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our Advantage Rich Experience Our products are sold all over Europe,North&South America, Sino-East, Asia and pacific area as well as Africa,we establish long term. Quality service Company cooperates with research institutes. We strictly con
Cas:147118-37-4
Min.Order:1 Kilogram
FOB Price: $15.0 / 50.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:147118-37-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:147118-37-4
Min.Order:1 Gram
FOB Price: $10.0 / 12.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
Empagliflozin CAS:864070-44-0 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermedia
Cas:147118-37-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Wuhan Zenuo Biological Medicine Technology Co Ltd
Product Name: 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl Synonyms: Z8(Rosuvastatin intermediate);4-(4-Fluorophenyl)-6-isopropyl-2-(N-Methyl-N-MethylsulfonylaMino)-5-pyriMidinecarboxaldehyde;4-(4-Flu
Cas:147118-37-4
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Hisunny Chemical Co.,Ltd
Best quality & Attractive price & Professional service; Trial & Pilot & Commercial Hisunny Chemical is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality intermediates, specia
Cas:147118-37-4
Min.Order:0
Negotiable
Type:Manufacturers
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:147118-37-4
Min.Order:1 Kilogram
FOB Price: $1.0 / 100000.0
Type:Lab/Research institutions
inquiryNanjing Fred Technology Co.,Ltd.
4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl is one of the most competitive products in our company, we can supply it with good quality and best price. Appearance:White solid Storage:Room temperature, Kee
Cas:147118-37-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:147118-37-4
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:147118-37-4
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:147118-37-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:147118-37-4
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
Methanesulfonamide, N-[4-(4-fluorophenyl)-5-formyl-6-(1-methylethyl)-2-pyrimidinyl]-N-methyl-Appearance:white powder Storage:Kept in a cool, dry and ventilated place Package:according to customers' requirements Application:research chemicals Transpor
Cas:147118-37-4
Min.Order:1 Gram
Negotiable
Type:Other
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:white to off white powder Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:10g/bag,or as your request Application:For medicine , pesticide inter
Cas:147118-37-4
Min.Order:1 Gram
Negotiable
Type:Trading Company
inquirySynthetic route
-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 9-azabicyclo<3.3.1>nonane-N-oxyl In acetonitrile at 20 - 50℃; for 2h; Sealed tube; | 99% |
| With fluorosulfonyl fluoride; potassium carbonate; dimethyl sulfoxide at 20℃; for 2h; Sealed tube; chemoselective reaction; | 99% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper(II) nitrate trihydrate In 2-methyltetrahydrofuran at 40℃; for 18h; Temperature; Reagent/catalyst; Solvent; | 99% |
-
-
1092844-00-2
4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidine-5-carbonitrile

-
-
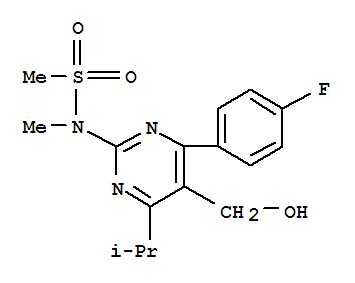
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With formic acid for 2h; Time; Reflux; | 95% |
| With diisobutylaluminium hydride In toluene at -5℃; for 1h; | 81% |
| Stage #1: 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidine-5-carbonitrile With diisobutylaluminium hydride In dichloromethane at 0 - 5℃; Inert atmosphere; Stage #2: With hydrogenchloride In dichloromethane; water at 0 - 30℃; | 80.3% |
-
-
916480-94-9
N-[5-[1,3]dioxolane-2-yl-4-(4-fluorophenyl)-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With acetic acid In water at 60 - 70℃; for 0.166667h; | 93% |
-
-
799842-07-2
N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide With sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide for 25h; Kornblum Oxydation; Stage #2: With acetic anhydride; dimethyl sulfoxide at 70℃; | 93% |
| Multi-step reaction with 2 steps 1: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux 2: acetic anhydride; dimethyl sulfoxide / 85 °C View Scheme | |
| Multi-step reaction with 2 steps 1: water / tetrahydrofuran / 6 h / Reflux 2: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme |

-
A
-
124752-23-4
tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: C29H40FN3O6S With ozone In methanol; dichloromethane at 25℃; for 5h; Stage #2: With thiourea In methanol; dichloromethane; water Temperature; Time; | A n/a B 82.2% |
-
-
147118-30-7
ethyl 2-(N-methyl-N-methanesulfonylamino)-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-carboxylic acid

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With lithium borohydride In tetrahydrofuran at 0 - 5℃; for 4h; | 80% |
| Multi-step reaction with 2 steps 1: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 2: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
1031246-43-1
4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 90 - 108℃; Temperature; | 72% |
| With trichlorophosphate at 90 - 108℃; | 72% |
-
-
1031246-43-1
4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide; trichlorophosphate at 90 - 108℃; Temperature; Concentration; | 72% |
-
-
147118-41-0
4-(4-fluorophenyl)-6-isopropyl-2-(methylamino)pyrimidine-5-carbaldehyde

-
-
124-63-0
methanesulfonyl chloride

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-(4-fluorophenyl)-6-isopropyl-2-(methylamino)pyrimidine-5-carbaldehyde With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: methanesulfonyl chloride In N,N-dimethyl-formamide at 0 - 20℃; for 3.5h; | 55% |
| Stage #1: 4-(4-fluorophenyl)-6-isopropyl-2-(methylamino)pyrimidine-5-carbaldehyde With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: methanesulfonyl chloride In N,N-dimethyl-formamide at 0 - 20℃; for 3.5h; Product distribution / selectivity; | 55% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; Product distribution / selectivity; | 30% |
-
-
1356998-77-0
N-(4-(4-fluorophenyl)-6-isopropyl-5-(prop-1-enyl)pyrimidin-2-yl)-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With oxone; water; sodium hydrogencarbonate; ruthenium(III) trichloride hydrate In acetonitrile at 20℃; for 24h; | 40% |
| With Oxone; sodium hydrogencarbonate; ruthenium(III) trichloride hydrate In water; acetonitrile at 20℃; for 24h; | 40% |
-
-
459-57-4
4-fluorobenzaldehyde

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 86.7 percent / piperidine, AcOH / benzene / Heating 2: 1.) HMPA, 2.) DDQ / 1.) 100 deg C, 22 h, 2.) benzene, 30 min 3: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 4: 46.9 g / ethanol / 1 h / Ambient temperature 5: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 6: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 7: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
122930-45-4
(E)-2-<(4-fluorophenyl)methylene>-4-methyl-3-oxopentanoic acid ethyl ester

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1.) HMPA, 2.) DDQ / 1.) 100 deg C, 22 h, 2.) benzene, 30 min 2: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 3: 46.9 g / ethanol / 1 h / Ambient temperature 4: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 5: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 6: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
7152-15-0
ethyl 4-methyl-3-oxo-pentanoate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 86.7 percent / piperidine, AcOH / benzene / Heating 2: 1.) HMPA, 2.) DDQ / 1.) 100 deg C, 22 h, 2.) benzene, 30 min 3: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 4: 46.9 g / ethanol / 1 h / Ambient temperature 5: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 6: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 7: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
147118-32-9
5-ethoxycarbonyl-6-(4'-fluorophenyl)-4-isopropyl-2-(methylamino)pyrimidine

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 2: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 3: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
147118-27-2
ethyl 4-(4-fluorophenyl)-2-(methylsulfanyl)-6-(propan-2-yl)-pyrimidine-5-carboxylate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 95.7 percent / m-CPBA / CHCl3 / Ambient temperature 2: 46.9 g / ethanol / 1 h / Ambient temperature 3: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 4: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 5: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
147118-28-3
ethyl 4-(4-fluorophenyl)-6-isopropyl-2-(methylsulfonyl)pyrimidine-5-carboxylate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 46.9 g / ethanol / 1 h / Ambient temperature 2: 1.) NaH / 1.) DMF, 30 min, 2.) DMF, RT, 2 h 3: 277 mg / DIBAL-H / toluene / 1 h / -74 °C 4: 71.2 percent / 4-methylmorpholine-N-oxide, tetrapropyl-ammonium perruthenate, molecular sieves 4 Angstroem / CH2Cl2 / 2 h View Scheme |
-
-
114433-94-2
1-(4-Fluorophenyl)-3-isopropylpropan-1,3-dione

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: potassium carbonate / acetone / 24 h / 20 °C 2: caesium carbonate / 2-methyltetrahydrofuran / 10 h / 40 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 5: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux 6: acetic anhydride; dimethyl sulfoxide / 85 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: potassium carbonate / acetone / 24 h / 20 °C 2.1: caesium carbonate / 2-methyltetrahydrofuran / 10 h / 40 °C 3.1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4.1: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 5.1: sodium hydrogencarbonate; dimethyl sulfoxide / sodium iodide / 25 h 5.2: 70 °C View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 4.17 h / 20 °C 2: sodium hydride / tetrahydrofuran; mineral oil / 48 h / 25 °C 3: triethylamine / dichloromethane / 17 h / -5 °C / Inert atmosphere 4: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 5: oxone; sodium hydrogencarbonate; water / ruthenium(III) trichloride hydrate / acetonitrile / 24 h / 20 °C View Scheme |
-
-
1207460-34-1
4-(4-fluorophenyl)-6-isopropyl-N,5-dimethylpyrimidin-2-amine

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 3: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux 4: acetic anhydride; dimethyl sulfoxide / 85 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2.1: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 3.1: sodium hydrogencarbonate; dimethyl sulfoxide / sodium iodide / 25 h 3.2: 70 °C View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 3: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere 4: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 3: water / tetrahydrofuran / 6 h / Reflux 4: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine / dichloromethane / 26 h / 0 - 20 °C 2: N-Bromosuccinimide / acetonitrile / 68 h / 20 °C / UV-irradiation 3: sodium hydrogencarbonate / acetonitrile / 4 h / Reflux 4: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme |
-
-
953776-62-0
N-(4-(4-fluorophenyl)-6-isopropyl-5-methylpyrimidin-2-yl)-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 2: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux 3: acetic anhydride; dimethyl sulfoxide / 85 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 2.1: sodium hydrogencarbonate; dimethyl sulfoxide / sodium iodide / 25 h 2.2: 70 °C View Scheme | |
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 2: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere 3: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme | |
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 2: water / tetrahydrofuran / 6 h / Reflux 3: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme | |
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide / acetonitrile / 68 h / 20 °C / UV-irradiation 2: sodium hydrogencarbonate / acetonitrile / 4 h / Reflux 3: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme |
-
-
1356998-74-7
2-allyl-1-(4-fluorophenyl)-4-methylpentane-1,3-dione

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydride / tetrahydrofuran; mineral oil / 48 h / 25 °C 2: triethylamine / dichloromethane / 17 h / -5 °C / Inert atmosphere 3: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 4: oxone; sodium hydrogencarbonate; water / ruthenium(III) trichloride hydrate / acetonitrile / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: tetrahydrofuran / 0.33 h / 20 °C 1.2: 48.16 h / 25 °C 2.1: triethylamine / dichloromethane / 17 h / -5 °C 3.1: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 4.1: sodium hydrogencarbonate; Oxone / ruthenium(III) trichloride hydrate / acetonitrile; water / 24 h / 20 °C View Scheme |
-
-
1356998-75-8
5-allyl-4-(4-fluorophenyl)-6-isopropyl-N-methylpyrimidin-2-amine

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 17 h / -5 °C / Inert atmosphere 2: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 3: oxone; sodium hydrogencarbonate; water / ruthenium(III) trichloride hydrate / acetonitrile / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 17 h / -5 °C 2: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 3: sodium hydrogencarbonate; Oxone / ruthenium(III) trichloride hydrate / acetonitrile; water / 24 h / 20 °C View Scheme |
-
-
1356998-76-9
N-(5-allyl-4-(4-fluorophenyl)-6-isopropylpyrimidin-2-yl)-N-methylmethanesulfonamide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 2: oxone; sodium hydrogencarbonate; water / ruthenium(III) trichloride hydrate / acetonitrile / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium hydroxide / Aliquat 336 / toluene / 24 h / 20 °C 2: sodium hydrogencarbonate; Oxone / ruthenium(III) trichloride hydrate / acetonitrile; water / 24 h / 20 °C View Scheme |
-
-
1354455-77-8
1-(4-fluorophenyl)-2-methyl-3-isopropylpropan-1,3-dione

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: caesium carbonate / 2-methyltetrahydrofuran / 10 h / 40 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 4: sodium hydrogencarbonate; water / acetonitrile / 4 h / Reflux 5: acetic anhydride; dimethyl sulfoxide / 85 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: caesium carbonate / 2-methyltetrahydrofuran / 10 h / 40 °C 2.1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3.1: N-Bromosuccinimide / acetonitrile / 0.5 h / UV-irradiation; Inert atmosphere; Sealed flow reactor 4.1: sodium hydrogencarbonate; dimethyl sulfoxide / sodium iodide / 25 h 4.2: 70 °C View Scheme | |
| Multi-step reaction with 5 steps 1: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere 5: acetic anhydride; dimethyl sulfoxide / 17 h / 85 °C View Scheme |
-
-
953776-62-0
N-(4-(4-fluorophenyl)-6-isopropyl-5-methylpyrimidin-2-yl)-N-methylmethanesulfonamide

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 2: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
799842-07-2
N-[5-(bromomethyl)-4-(4-fluorophenyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide at 20℃; for 71h; Kornblum oxidation; Inert atmosphere; |
-
-
1354455-77-8
1-(4-fluorophenyl)-2-methyl-3-isopropylpropan-1,3-dione

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: caesium carbonate / 2-methyltetrahydrofuran / 24 h / 70 °C 2: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 3: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 4: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
1207460-34-1
4-(4-fluorophenyl)-6-isopropyl-N,5-dimethylpyrimidin-2-amine

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 2: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 3: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
114433-94-2
1-(4-Fluorophenyl)-3-isopropylpropan-1,3-dione

-
A
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

-
B
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 48 h / 20 °C / Inert atmosphere 2: potassium tert-butylate / tert-butyl alcohol / 24 h / 70 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 5: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetone / 48 h / 20 °C / Inert atmosphere 2: caesium carbonate / 2-methyltetrahydrofuran / 24 h / 70 °C 3: triethylamine / dichloromethane / 8 h / -5 °C / Inert atmosphere 4: N-Bromosuccinimide / acetonitrile / 16 h / 20 °C / Inert atmosphere; UV-irradiation 5: sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide / 71 h / 20 °C / Inert atmosphere View Scheme |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: dmap; ammonia / toluene; water / 0 - 35 °C / Inert atmosphere 2.1: copper(l) chloride; sulfuric acid / methanol / 30 - 70 °C / Inert atmosphere; Reflux 3.1: potassium carbonate; tert.-butylhydroperoxide; copper(II) choride dihydrate / dichloromethane / 25 - 30 °C / Inert atmosphere 4.1: trichlorophosphate / dichloromethane / 0 - 105 °C / Inert atmosphere 5.1: potassium carbonate / toluene / 25 - 115 °C / Inert atmosphere; Reflux 6.1: diisobutylaluminium hydride / dichloromethane / 0 - 5 °C / Inert atmosphere 6.2: 0 - 30 °C View Scheme |
-
-
147118-35-2
methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-38-5
7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonamido)-pyrimidin-5-yl)-(3R)-3-tert-butyldimethylsiloxy-5-oxo-(E)-6-heptenoic acid methyl ester

| Conditions | Yield |
|---|---|
| In toluene for 30h; Wittig condensation; Heating / reflux; | 100% |
| Stage #1: methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate; N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In acetonitrile for 10 - 12h; Heating / reflux; Stage #2: With acetic acid In di-isopropyl ether; water at 20℃; for 0.25h; Stage #3: With water; sodium hydrogencarbonate In di-isopropyl ether Product distribution / selectivity; | 100% |
| In acetonitrile for 10 - 12h; Product distribution / selectivity; Heating / reflux; | 100% |
-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
147118-36-3
N-[4-(4-fluorophenyl)-5-(hydroxymethyl)-6-(propan-2-yl)pyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 5 - 25℃; for 2h; | 98% |
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 5 - 10℃; | |
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 0 - 20℃; for 1h; |
-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
1024024-26-7
methyl 3-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]-(2E)-propenoate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20 - 81℃; | 96% |
-
-
79-19-6
thiosemicarbazide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide; allyl halide In ethanol for 3h; Reflux; Stage #2: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In ethanol for 5h; Reflux; | 93% |


-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 3h; Wittig Olefination; | 92.9% |
-
-
79-19-6
thiosemicarbazide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide; isopentyl halide In ethanol for 3h; Reflux; Stage #2: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In ethanol for 6h; Reflux; | 92% |

-
-
380460-37-7
[(4R,6S)-2,2-dimethyl-6-(1-phenyl-1H-tetrazole-5-sulfonylmethyl)[1,3]dioxan-4-yl]acetic acid tert-butyl ester

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
289042-12-2
2-[(4R,6S)-6-[(E)-2-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(isopropyl)pyrimidin-5-yl]vinyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert butyl ester

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; water at -15 - 42℃; for 1h; Temperature; Inert atmosphere; | 91% |
| With sodium hydride In tetrahydrofuran at 0 - 35℃; Solvent; Temperature; Reagent/catalyst; | 120 g |

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 3h; Wittig Olefination; | 90.6% |
-
-
1622874-01-4
(R)-methyl 3-(tert-butyldimethylsilyloxy)-5-oxohexanoate

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide With tin(IV) chloride In tetrahydrofuran; dichloromethane at 0℃; for 0.25h; Inert atmosphere; Stage #2: (R)-methyl 3-(tert-butyldimethylsilyloxy)-5-oxohexanoate With 4-methyl-morpholine In tetrahydrofuran; dichloromethane at 0℃; for 3h; Reagent/catalyst; Inert atmosphere; | 87% |
-
-
79-19-6
thiosemicarbazide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide; benzyl halide In ethanol for 3h; Reflux; Stage #2: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In ethanol for 6h; Reflux; | 85% |

-
-
79-19-6
thiosemicarbazide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide; n-heptyl halide In ethanol for 3h; Reflux; Stage #2: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In ethanol for 6h; Reflux; | 85% |


-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; tert-butyl alcohol at -40℃; for 4h; | 85% |

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
289042-12-2
2-[(4R,6S)-6-[(E)-2-[4-(4-fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(isopropyl)pyrimidin-5-yl]vinyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert butyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 42℃; Inert atmosphere; | 84.5% |
-
-
79-19-6
thiosemicarbazide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide; isopropyl halide In ethanol for 3h; Reflux; Stage #2: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In ethanol for 5.5h; Reflux; | 83% |

-
-
106-95-6
allyl bromide

-
-
147118-37-4
N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

-
-
1352138-41-0
N-[4-(4-fluorophenyl)-5-(1-hydroxybut-3-enyl)-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: allyl bromide With magnesium In diethyl ether for 0.5h; Inert atmosphere; Stage #2: N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl]-N-methylmethanesulfonamide In tetrahydrofuran; diethyl ether at -5 - 5℃; Stage #3: With hydrogenchloride; water In tetrahydrofuran; diethyl ether for 0.5h; | 81% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View