Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:207679-81-0
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:207679-81-0
Min.Order:1
Negotiable
Type:Other
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:207679-81-0
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:207679-81-0
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryWin-Win chemical Co.Ltd
Stock products, own laboratory Appearance:Off-White to Pale Yellow Solid Storage:-20°C Freezer Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:shanghai
Cas:207679-81-0
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:207679-81-0
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
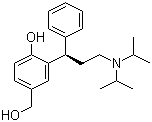
Name: 3-[(1R)-3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-hydroxybenzenemethanol Iterm: Pharmaceutical intermediates CAS: 207679-81-0 structure:
Cas:207679-81-0
Min.Order:5 Metric Ton
FOB Price: $1.0 / 2.0
Type:Other
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:207679-81-0
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
(R)-5-HydroxyMethyl Tolterodine CAS:207679-81-0 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality
Cas:207679-81-0
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:207679-81-0
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryXiamen Hisunny Chemical Co.,Ltd
Best quality & Attractive price & Professional service; Trial & Pilot & Commercial Hisunny Chemical is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality intermediates, specia
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Manufacturers
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:207679-81-0
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
our strengths: 1: Fast and guaranteed shipment (TNT;EMS;FEDEX;DHL;UPS;EUB, special line) 2: Various payment items accepted (Btc; MoneyGram; WU) 3: Valued package (Paraffin coating; Double aluminum foil bag; Vacuum packaging) 4: Efficient delivery
Cas:207679-81-0
Min.Order:1 Gram
Negotiable
Type:Other
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:207679-81-0
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:207679-81-0
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:207679-81-0
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:207679-81-0
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryLuyunjia Chemistry Xiamen Limited
Luyunjia Chemistry Xiamen Limited is a high-tech company specializing in R&D and manufacturing of photoinitiators monomers and oligomers, UV absorbers and pharmaceutical intermediates, and pharmaceutical intermediates of generic drug. and has a h
Cas:207679-81-0
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Hebei Mojin Biotechnology Co.,Ltd
1, High quality with competitive price:2, Fast and safe delivery3.Excellent pre-sales and after-sales service4. Well-trained and professional technologist and sales with rich experience in the field for 5-10 yearsAppearance:see detailed specification
GIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Dingyan Chem Co., Ltd
R & D enterprises have their own stock in stock Package:1kg Application:pharmaceutical intermediates
HANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:white crystalline powder Storage:Sealed in a cool ,dry and microtherm place , avoid light . Package: 1g/bag, 5g/bag, 10g/bag, 100g/bag, 1kg/bag or as per your request Application:It
Cas:207679-81-0
Min.Order:1 Gram
Negotiable
Type:Trading Company
inquiryAecochem Corp.
Our clients, like BASF,CHEMO,Brenntag,ASR,Evonik,Merck and etc.Appearance:COA Storage:in stock Application:MSDS/TDS
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Manufacturers
inquiryXian Changyue Biological Technology Co., Ltd.
best seller Application:API
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Manufacturers
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryGuangdong Juda Chemical Industrial Co.,Limited
Factory supply high purity low priceAppearance:solid or liquid Storage:sealed in cool and dry place Package:As customer's requested Application:Pharma Intermediate Transportation:by courier/air/sea Port:Any port in China
Wuhan Circle Star Chem-medical Technology co.,Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:white powder Storage:Keep it in dry,shady and cool place Package:25kg,50kg,180kg,200kg,250kg,1000kg,customization Application:Pharma;Industry;Agricultural;chemical reaserch Tran
Cas:207679-81-0
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySynthetic route

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; toluene at 50℃; for 6h; | 85% |
| With potassium carbonate In water; toluene at 50℃; for 6h; Inert atmosphere; | 85% |
| With potassium carbonate In water; toluene at 50℃; for 1h; | 81% |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: (R)-(-)-3-(3-diisopropylamino-1-phenylpropyl)-4-hydroxybenzoic acid methyl ester With sodium hydroxide In methanol at 0 - 35℃; Inert atmosphere; Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran at 5 - 25℃; for 4h; Stage #3: With water; sodium hydroxide In tetrahydrofuran at 0 - 5℃; for 3h; | 98% |
| Stage #1: (R)-(-)-3-(3-diisopropylamino-1-phenylpropyl)-4-hydroxybenzoic acid methyl ester With sodium hydroxide In methanol at 0 - 35℃; Inert atmosphere; Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran at 5 - 25℃; Stage #3: With water; sodium hydroxide In tetrahydrofuran for 3h; | 98% |
| Stage #1: (R)-(-)-3-(3-diisopropylamino-1-phenylpropyl)-4-hydroxybenzoic acid methyl ester With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 3.5h; Stage #2: With ammonium chloride In tetrahydrofuran at -10℃; | 95% |
| With sodium bis(2-methoxyethoxy)aluminium dihydride In tetrahydrofuran at 0 - 5℃; for 2h; Inert atmosphere; |
-
-
156755-37-2
{4-(benzyloxy)-3-[(1R)-3-(dipropan-2-ylamino)-1-phenylpropyl]phenyl}methanol

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With hydrogen; 5% Pd(II)/C(eggshell) In methanol at 50 - 55℃; | 98% |
| With hydrogen; nickel In methanol at 20℃; for 4 - 5h; | 96.5% |
| With hydrogen In methanol under 3000.3 - 3750.38 Torr; | 72.5% |
-
-
1294517-15-9
methyl (R)-(-)-3-(3-diisopropylamino-1-phenyl-propyl)-4-hydroxy-benzoate 2,3-dibenzoyl-D-tartaric acid salt

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: methyl (R)-(-)-3-(3-diisopropylamino-1-phenyl-propyl)-4-hydroxy-benzoate 2,3-dibenzoyl-D-tartaric acid salt With sodium hydrogencarbonate In ethyl acetate Inert atmosphere; Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 3.5h; Stage #3: With ammonium chloride In tetrahydrofuran at -10 - 20℃; for 0.5h; | 95% |
| Multi-step reaction with 2 steps 1.1: sodium hydrogencarbonate / ethyl acetate; water / Inert atmosphere 2.1: sodium hydroxide / methanol / 0 - 35 °C / Inert atmosphere 2.2: 5 - 25 °C 2.3: 3 h View Scheme |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 20℃; for 18h; | 55% |
-
-
1449220-60-3
D-(+)-menthyl 3-(3-N,N'-diisopropylamino-1(R)-phenyl-propyl)-4-hydroxy-benzoate hydrochloride

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: D-(+)-menthyl 3-(3-N,N'-diisopropylamino-1(R)-phenyl-propyl)-4-hydroxy-benzoate hydrochloride With water; sodium hydroxide In dichloromethane pH=7 - 8; Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 25℃; | 83% |
| Stage #1: D-(+)-menthyl 3-(3-N,N'-diisopropylamino-1(R)-phenyl-propyl)-4-hydroxy-benzoate hydrochloride With sodium hydroxide In dichloromethane; water pH=7 - 8; Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 25℃; | 83% |
-
-
960373-33-5
(R)-6-hydroxymethyl-4-phenylchroman-2-(R)-ol

-
-
108-18-9
diisopropylamine

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: (R)-6-hydroxymethyl-4-phenylchroman-2-(R)-ol; diisopropylamine With hydrogen; palladium on activated charcoal In methanol at 20℃; under 3000.3 Torr; for 18h; Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran Stage #3: With water In tetrahydrofuran |
-
-
1333234-72-2
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol (R)-2-acetoxy(phenyl)acetate

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 50℃; for 2h; | 70% |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: (R)-[4-benzyloxy-3-(3-diisopropylamino-1-phenyl-propyl)-phenyl]-methanol fumarate With sodium carbonate; sodium hydroxide In dichloromethane; water Stage #2: With hydrogen; Raney nickel In methanol | 59% |
-
-
200801-70-3
RS-N,N-diisopropyl-3-(2-hydroxy-5-(hydroxymethyl)phenyl)-3-phenylpropylamine

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: RS-N,N-diisopropyl-3-(2-hydroxy-5-(hydroxymethyl)phenyl)-3-phenylpropylamine With D-Malic acid In di-isopropyl ether; isopropyl alcohol at 20 - 80℃; Stage #2: With sodium hydrogencarbonate In water pH=10; Product distribution / selectivity; | 40.2% |
| Multi-step reaction with 2 steps 1: tert-Amyl alcohol / 25 - 70 °C 2: potassium carbonate / toluene; water / 1 h / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: tetrahydrofuran / 10 - 55 °C 2: potassium carbonate / toluene; water / 50 - 55 °C View Scheme |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; toluene at 50 - 55℃; |
-
-
1390644-47-9
(R)-3-(3-(diisopropylamino)-1-phenylpropyl)-4-hydroxybenzyl acetate

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With water; lithium hydroxide In methanol at 25 - 35℃; for 4h; |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; tetrakis(triphenylphosphine) palladium(0) In methanol at 25 - 30℃; for 2h; |
-
-
214601-12-4
(R)-N,N-diisopropyl-3-phenyl-3-(5-formyl-2-hydroxyphenyl)propyl-1-amine

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol Product distribution / selectivity; | 74% |
| Stage #1: (R)-N,N-diisopropyl-3-phenyl-3-(5-formyl-2-hydroxyphenyl)propyl-1-amine With lithium aluminium tetrahydride In tetrahydrofuran for 2h; Stage #2: With water; sodium hydrogencarbonate In tetrahydrofuran at 0℃; Product distribution / selectivity; | 73% |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 92% |
-
-
99-76-3
methyl 4-hydroxylbenzoate

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: methanesulfonic acid / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 20 - 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate / Inert atmosphere 3.2: 3.5 h / 20 °C 3.3: 0.5 h / -10 - 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / water / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate / Inert atmosphere 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 20 °C 4.2: -10 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 20 - 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate; water / Inert atmosphere 4.1: sodium hydroxide / methanol / 0 - 35 °C / Inert atmosphere 4.2: 4 h / 5 - 25 °C 4.3: 3 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate; water / Inert atmosphere 4.1: sodium hydroxide / methanol / 0 - 35 °C / Inert atmosphere 4.2: 5 - 25 °C 4.3: 3 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1-methyl-piperazine / toluene / 25 - 110 °C 1.2: 5 h / 60 - 65 °C 2.1: hydrogen / 5%-palladium/activated carbon / methanol / 25 - 50 °C / 10343.2 Torr / Autoclave 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 0 - 30 °C / Inert atmosphere 4.1: D-Malic acid / isopropyl alcohol; di-isopropyl ether / 20 - 80 °C 4.2: pH 10 View Scheme |
-
-
906532-26-1
3-(N,N-diisopropylamino)-1-phenylpropan-1-ol

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: methanesulfonic acid / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 20 - 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate / Inert atmosphere 3.2: 3.5 h / 20 °C 3.3: 0.5 h / -10 - 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / water / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate / Inert atmosphere 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 20 °C 4.2: -10 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 20 - 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate; water / Inert atmosphere 4.1: sodium hydroxide / methanol / 0 - 35 °C / Inert atmosphere 4.2: 4 h / 5 - 25 °C 4.3: 3 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanesulfonic acid / 50 - 55 °C / Inert atmosphere 2.1: ethanol / 60 °C / Inert atmosphere 3.1: sodium hydrogencarbonate / ethyl acetate; water / Inert atmosphere 4.1: sodium hydroxide / methanol / 0 - 35 °C / Inert atmosphere 4.2: 5 - 25 °C 4.3: 3 h View Scheme | |
| Multi-step reaction with 4 steps 1: perchloric acid / dichloromethane; water / 13 h / 40 °C / Inert atmosphere 2: sodium tetrahydroborate; calcium chloride / ethanol / 3 h / -10 - 20 °C / Inert atmosphere 3: tert-Amyl alcohol / 17 h / 20 - 70 °C / Inert atmosphere 4: potassium carbonate / water; toluene / 6 h / 50 °C / Inert atmosphere View Scheme |
-
-
156755-35-0
methyl 4-(benzyloxy)-3-[(1R)-3-(dipropan-2-ylamino)-1-phenylpropyl]benzoate

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 2: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 3 h / Inert atmosphere 2: hydrogen / Raney nickel / methanol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 5 h / 0 - 20 °C / Inert atmosphere 1.2: 0 - 5 °C 2.1: hydrogen / 5%-palladium/activated carbon / methanol / 2 h / 25 - 35 °C / Autoclave 2.2: 25 - 35 °C View Scheme |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: triethylamine / dichloromethane / 12 h / 25 - 30 °C 2.1: acetonitrile / 30 h / 95 - 100 °C / autoclave; Sealed tube 3.1: isopropyl alcohol / 15 h / 25 - 86 °C 4.1: sodium hydroxide / water 5.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 5.2: 1 h / -65 - -60 °C 6.1: thionyl chloride / 10 °C / Reflux 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 8.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: pyridine / dichloromethane / 5 - 30 °C / Industry scale 2.1: water / 75 - 78 °C / Industry scale 3.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 50 - 60 °C 3.2: -70 - -60 °C 3.3: -50 °C 4.1: borane-dimethyl sulfide complex / tetrahydrofuran / 25 - 50 °C 4.2: 0.5 h / 10 - 15 °C / Industry scale 5.1: raney nickel / methanol / 30 - 35 °C / Autoclave 6.1: tetrahydrofuran / 10 - 55 °C 7.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: pyridine / dichloromethane / 5 - 30 °C / Industry scale 1.2: 5 - 30 °C / Industry scale 2.1: water / 75 - 78 °C / Industry scale 3.1: ethyl bromide; magnesium / iodine / tetrahydrofuran / 50 - 60 °C 3.2: -70 - -60 °C 4.1: dimethylsulfide borane complex / tetrahydrofuran / 25 - 50 °C 5.1: methanol / 30 - 35 °C 6.1: tetrahydrofuran / 10 - 55 °C 7.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: isopropyl alcohol / 15 h / 25 - 86 °C 2.1: sodium hydroxide / water 3.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 3.2: 1 h / -65 - -60 °C 4.1: thionyl chloride / 10 °C / Reflux 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 6.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 50 - 60 °C 1.2: -70 - -60 °C 1.3: -50 °C 2.1: borane-dimethyl sulfide complex / tetrahydrofuran / 25 - 50 °C 2.2: 0.5 h / 10 - 15 °C / Industry scale 3.1: raney nickel / methanol / 30 - 35 °C / Autoclave 4.1: tetrahydrofuran / 10 - 55 °C 5.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: ethyl bromide; magnesium / iodine / tetrahydrofuran / 50 - 60 °C 1.2: -70 - -60 °C 2.1: dimethylsulfide borane complex / tetrahydrofuran / 25 - 50 °C 3.1: methanol / 30 - 35 °C 4.1: tetrahydrofuran / 10 - 55 °C 5.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme |
-
-
156755-23-6
6-bromo-4-phenyl-3,4-dihydro-2H-chromen-2-one

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: potassium carbonate; sodium iodide / acetone / Reflux 2.1: sodium tetrahydroborate / 1,2-dimethoxyethane / 0.17 h 2.2: 3 h / 10 °C 3.1: triethylamine / dichloromethane / 12 h / 25 - 30 °C 4.1: acetonitrile / 30 h / 95 - 100 °C / autoclave; Sealed tube 5.1: isopropyl alcohol / 15 h / 25 - 86 °C 6.1: sodium hydroxide / water 7.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 7.2: 1 h / -65 - -60 °C 8.1: thionyl chloride / 10 °C / Reflux 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 10.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 35 °C / Industry scale 1.2: 5 - 30 °C / pH 1 - 2 / Industry scale 2.1: potassium carbonate / acetone / 20 - 25 °C / Industry scale 2.2: 50 - 60 °C 3.1: pyridine / dichloromethane / 5 - 30 °C / Industry scale 4.1: water / 75 - 78 °C / Industry scale 5.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 50 - 60 °C 5.2: -70 - -60 °C 5.3: -50 °C 6.1: borane-dimethyl sulfide complex / tetrahydrofuran / 25 - 50 °C 6.2: 0.5 h / 10 - 15 °C / Industry scale 7.1: raney nickel / methanol / 30 - 35 °C / Autoclave 8.1: tetrahydrofuran / 10 - 55 °C 9.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme | |
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 35 °C / Industry scale 1.2: 0 - 5 °C / Industry scale; Reflux 1.3: 0.5 h / 25 - 30 °C / pH 1 - 2 / Industry scale 2.1: potassium carbonate / acetone / 20 - 25 °C / Industry scale 2.2: 50 - 60 °C / Industry scale 3.1: pyridine / dichloromethane / 5 - 30 °C / Industry scale 3.2: 5 - 30 °C / Industry scale 4.1: water / 75 - 78 °C / Industry scale 5.1: ethyl bromide; magnesium / iodine / tetrahydrofuran / 50 - 60 °C 5.2: -70 - -60 °C 6.1: dimethylsulfide borane complex / tetrahydrofuran / 25 - 50 °C 7.1: methanol / 30 - 35 °C 8.1: tetrahydrofuran / 10 - 55 °C 9.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme |
-
-
250214-37-0
(+/-)-toluene-4-sulphonic acid 3-(2-benzyloxy-5-bromophenyl)-3-phenylpropyl ester

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: acetonitrile / 30 h / 95 - 100 °C / autoclave; Sealed tube 2.1: isopropyl alcohol / 15 h / 25 - 86 °C 3.1: sodium hydroxide / water 4.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 4.2: 1 h / -65 - -60 °C 5.1: thionyl chloride / 10 °C / Reflux 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 7.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: water / 75 - 78 °C / Industry scale 2.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 50 - 60 °C 2.2: -70 - -60 °C 2.3: -50 °C 3.1: borane-dimethyl sulfide complex / tetrahydrofuran / 25 - 50 °C 3.2: 0.5 h / 10 - 15 °C / Industry scale 4.1: raney nickel / methanol / 30 - 35 °C / Autoclave 5.1: tetrahydrofuran / 10 - 55 °C 6.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: water / 75 - 78 °C / Industry scale 2.1: ethyl bromide; magnesium / iodine / tetrahydrofuran / 50 - 60 °C 2.2: -70 - -60 °C 3.1: dimethylsulfide borane complex / tetrahydrofuran / 25 - 50 °C 4.1: methanol / 30 - 35 °C 5.1: tetrahydrofuran / 10 - 55 °C 6.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme |
-
-
156755-24-7
methyl 3-[2-(benzyloxy)-5-bromophenyl]-3-phenylpropanoate

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / 1,2-dimethoxyethane / 0.17 h 1.2: 3 h / 10 °C 2.1: triethylamine / dichloromethane / 12 h / 25 - 30 °C 3.1: acetonitrile / 30 h / 95 - 100 °C / autoclave; Sealed tube 4.1: isopropyl alcohol / 15 h / 25 - 86 °C 5.1: sodium hydroxide / water 6.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 6.2: 1 h / -65 - -60 °C 7.1: thionyl chloride / 10 °C / Reflux 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 9.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: aluminum (III) chloride; sodium tetrahydroborate / 1,2-dimethoxyethane / 3.5 h / 0 - 10 °C 1.2: 0 - 5 °C 2.1: triethylamine / dichloromethane / 0.5 h / 20 - 30 °C 3.1: 25 - 112 °C / Autoclave 4.1: isopropyl alcohol / 14 h / 25 - 80 °C 5.1: sodium hydroxide / water / 0 - 35 °C / pH 9 6.1: magnesium / iodine / tetrahydrofuran / 1 h / -70 - 70 °C 6.2: -5 - 35 °C 6.3: pH 1 - 2 7.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 0.25 h / 0 - 5 °C 8.1: hydrogen / 5%-palladium/activated carbon / methanol / 2 h / 25 - 35 °C / Autoclave 8.2: 25 - 35 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 4 h / 5 - 10 °C / Inert atmosphere 2.1: dmap; triethylamine / dichloromethane / 2 h / 0 - 5 °C 3.1: acetonitrile / 32 h / 95 - 100 °C / autoclave; Inert atmosphere 4.1: isopropyl alcohol / 30 - 55 °C 5.1: sodium hydroxide; water / dichloromethane / 0.25 h / 10 - 20 °C / pH 11.5 5.2: 4 h / 25 - 55 °C / Inert atmosphere 5.3: -70 - -10 °C / Inert atmosphere 6.1: thionyl chloride / 5 h / 40 - 45 °C 7.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 4 h / 0 - 5 °C / Inert atmosphere 8.1: hydrogen / 5%-palladium/activated carbon / methanol / 8 h / 25 - 30 °C / 2206.72 - 2942.29 Torr View Scheme |
-
-
108-18-9
diisopropylamine

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate; potassium iodide / toluene; methanol / 20 h / Reflux 2.1: methanesulfonic acid / 3 h / 100 °C 3.1: sodium tetrahydroborate / methanol / 2 h / 0 °C 3.2: 0.08 h 3.3: pH 8 4.1: tert-Amyl alcohol / 25 - 70 °C 5.1: potassium carbonate / toluene; water / 1 h / 50 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: potassium carbonate; potassium iodide / toluene; methanol / 20 h / Reflux 2.1: methanesulfonic acid / 20 h / 120 °C 2.2: pH 7 2.3: 21 h / 100 °C 3.1: sodium tetrahydroborate / methanol / 2 h / 0 °C 3.2: 0.08 h 3.3: pH 8 4.1: tert-Amyl alcohol / 25 - 70 °C 5.1: potassium carbonate / toluene; water / 1 h / 50 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: potassium carbonate; potassium iodide / toluene; methanol / 20 h / Reflux 2.1: methanesulfonic acid / 23 h / 130 °C 2.2: pH 7 2.3: 4 h / 78 °C 3.1: sodium tetrahydroborate / methanol / 2 h / 0 °C 3.2: 0.08 h 3.3: pH 8 4.1: tert-Amyl alcohol / 25 - 70 °C 5.1: potassium carbonate / toluene; water / 1 h / 50 °C View Scheme |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: borane-dimethyl sulfide complex / tetrahydrofuran / 25 - 50 °C 1.2: 0.5 h / 10 - 15 °C / Industry scale 2.1: raney nickel / methanol / 30 - 35 °C / Autoclave 3.1: tetrahydrofuran / 10 - 55 °C 4.1: potassium carbonate / toluene; water / 50 - 55 °C View Scheme | |
| Multi-step reaction with 4 steps 1: dimethylsulfide borane complex / tetrahydrofuran / 25 - 50 °C 2: methanol / 30 - 35 °C 3: tetrahydrofuran / 10 - 55 °C 4: potassium carbonate / toluene; water / 50 - 55 °C View Scheme | |
| Multi-step reaction with 6 steps 1: ammonia / ethyl acetate; water / pH 8 - 9 2: methanol / 50 - 65 °C 3: ammonia / water / pH 8 - 9 4: sulfuric acid / 20 °C / Reflux 5: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 3 h / Inert atmosphere 6: hydrogen / Raney nickel / methanol / 20 °C View Scheme |
-
-
194482-44-5
Tolterodine acid

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: Tolterodine acid With lithium aluminium tetrahydride In tetrahydrofuran for 2h; Stage #2: With water; sodium hydrogencarbonate In tetrahydrofuran at 0℃; Product distribution / selectivity; |
-
-
106-41-2
4-bromo-phenol

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: sulfuric acid / 120 - 125 °C 2.1: potassium carbonate; sodium iodide / acetone / Reflux 3.1: sodium tetrahydroborate / 1,2-dimethoxyethane / 0.17 h 3.2: 3 h / 10 °C 4.1: triethylamine / dichloromethane / 12 h / 25 - 30 °C 5.1: acetonitrile / 30 h / 95 - 100 °C / autoclave; Sealed tube 6.1: isopropyl alcohol / 15 h / 25 - 86 °C 7.1: sodium hydroxide / water 8.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 8.2: 1 h / -65 - -60 °C 9.1: thionyl chloride / 10 °C / Reflux 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 11.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 10 steps 1.1: sulfuric acid / 4 h / 25 - 125 °C 2.1: potassium carbonate; sodium iodide / acetone / 2 h / 25 - 60 °C 3.1: aluminum (III) chloride; sodium tetrahydroborate / 1,2-dimethoxyethane / 3.5 h / 0 - 10 °C 3.2: 0 - 5 °C 4.1: triethylamine / dichloromethane / 0.5 h / 20 - 30 °C 5.1: 25 - 112 °C / Autoclave 6.1: isopropyl alcohol / 14 h / 25 - 80 °C 7.1: sodium hydroxide / water / 0 - 35 °C / pH 9 8.1: magnesium / iodine / tetrahydrofuran / 1 h / -70 - 70 °C 8.2: -5 - 35 °C 8.3: pH 1 - 2 9.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 0.25 h / 0 - 5 °C 10.1: hydrogen / 5%-palladium/activated carbon / methanol / 2 h / 25 - 35 °C / Autoclave 10.2: 25 - 35 °C View Scheme | |
| Multi-step reaction with 10 steps 1.1: sulfuric acid / 12 h / 120 - 125 °C 2.1: potassium carbonate; sodium iodide / acetone / 25 - 30 °C 2.2: 5.5 h / 15 °C / Reflux 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 4 h / 5 - 10 °C / Inert atmosphere 4.1: dmap; triethylamine / dichloromethane / 2 h / 0 - 5 °C 5.1: acetonitrile / 32 h / 95 - 100 °C / autoclave; Inert atmosphere 6.1: isopropyl alcohol / 30 - 55 °C 7.1: sodium hydroxide; water / dichloromethane / 0.25 h / 10 - 20 °C / pH 11.5 7.2: 4 h / 25 - 55 °C / Inert atmosphere 7.3: -70 - -10 °C / Inert atmosphere 8.1: thionyl chloride / 5 h / 40 - 45 °C 9.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 4 h / 0 - 5 °C / Inert atmosphere 10.1: hydrogen / 5%-palladium/activated carbon / methanol / 8 h / 25 - 30 °C / 2206.72 - 2942.29 Torr View Scheme | |
| Multi-step reaction with 10 steps 1.1: sulfuric acid / 12 h / 120 - 125 °C 2.1: potassium carbonate; sodium iodide / acetone / 25 - 30 °C 2.2: 5.5 h / 15 °C / Reflux 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 4 h / 5 - 10 °C / Inert atmosphere 4.1: dmap; triethylamine / dichloromethane / 2 h / 0 - 5 °C 5.1: acetonitrile / 32 h / 95 - 100 °C / autoclave; Inert atmosphere 6.1: isopropyl alcohol / 30 - 55 °C 7.1: sodium hydroxide; water / dichloromethane / 0.25 h / 10 - 20 °C / pH 11.5 7.2: 4 h / 25 - 55 °C / Inert atmosphere 7.3: -70 - -10 °C / Inert atmosphere 8.1: thionyl chloride / 5 h / 40 - 45 °C 9.1: hydrogen / 5%-palladium/activated carbon / ethyl acetate / 18 h / 25 - 30 °C / 2206.72 - 2942.29 Torr / autoclave 10.1: sodium bis(2-methoxyethoxy)aluminium dihydride / tetrahydrofuran / 2 h / 0 - 5 °C / Inert atmosphere View Scheme |
-
-
156755-33-8
(R)-4-(benzyloxy)-3-(3-(diisopropylamino)-1-phenylpropyl)benzoic acid hydrochloride

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: thionyl chloride / 10 °C / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 3: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 0.25 h / 0 - 5 °C 2.1: hydrogen / 5%-palladium/activated carbon / methanol / 2 h / 25 - 35 °C / Autoclave 2.2: 25 - 35 °C View Scheme | |
| Multi-step reaction with 3 steps 1: thionyl chloride / 5 h / 40 - 45 °C 2: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 4 h / 0 - 5 °C / Inert atmosphere 3: hydrogen / 5%-palladium/activated carbon / methanol / 8 h / 25 - 30 °C / 2206.72 - 2942.29 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: thionyl chloride / 5 h / 40 - 45 °C 2: hydrogen / 5%-palladium/activated carbon / ethyl acetate / 18 h / 25 - 30 °C / 2206.72 - 2942.29 Torr / autoclave 3: sodium bis(2-methoxyethoxy)aluminium dihydride / tetrahydrofuran / 2 h / 0 - 5 °C / Inert atmosphere View Scheme |
-
-
950773-38-3
R-(-)-[3-(2-benzyloxy-5-bromophenyl)-3-phenylpropyl]diisopropylamine

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: ethyl bromide; iodine; magnesium / tetrahydrofuran / 1 h / 55 °C / Reflux 1.2: 1 h / -65 - -60 °C 2.1: thionyl chloride / 10 °C / Reflux 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 14 h / 25 - 30 °C 4.1: hydrogen / 5% Pd(II)/C(eggshell) / methanol / 50 - 55 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: magnesium / iodine / tetrahydrofuran / 1 h / -70 - 70 °C 1.2: -5 - 35 °C 1.3: pH 1 - 2 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 0.25 h / 0 - 5 °C 3.1: hydrogen / 5%-palladium/activated carbon / methanol / 2 h / 25 - 35 °C / Autoclave 3.2: 25 - 35 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: iodine; magnesium; ethyl bromide / tetrahydrofuran / 2 h / 60 - 65 °C / Inert atmosphere 1.2: 1 h / -70 - -50 °C 2.1: thionyl chloride / 0 - 65 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 30 °C 4.1: hydrogen; palladium 10% on activated carbon / isopropyl alcohol / 25 - 30 °C / 2068.65 - 2585.81 Torr / Autoclave View Scheme |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 1-methyl-piperazine / toluene / 25 - 110 °C 1.2: 5 h / 60 - 65 °C 2.1: hydrogen / 5%-palladium/activated carbon / methanol / 25 - 50 °C / 10343.2 Torr / Autoclave 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 0 - 30 °C / Inert atmosphere 4.1: D-Malic acid / isopropyl alcohol; di-isopropyl ether / 20 - 80 °C 4.2: pH 10 View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1-methyl-piperazine / toluene / Heating 1.2: 5 h / Reflux 2.1: methanol / 1.25 h / 40 °C / Inert atmosphere 2.2: 20 h / 40 °C / 5948.09 Torr / Inert atmosphere 3.1: tert-Amyl alcohol / 70 °C / Resolution of racemate; Large scale reaction 4.1: potassium carbonate / water; toluene / 6 h / 50 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1-methyl-piperazine / toluene / Heating 1.2: 5 h / Reflux 2.1: 5%-palladium/activated carbon; hydrogen / tert-Amyl alcohol / 80 °C / 5171.62 Torr 3.1: tert-Amyl alcohol / 70 °C / Resolution of racemate; Large scale reaction 4.1: potassium carbonate / water; toluene / 6 h / 50 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1-methyl-piperazine / toluene / Heating 1.2: 5 h / Reflux 2.1: titanium(IV)isopropoxide / tetrahydrofuran / 1 h / Reflux 2.2: 20 °C 3.1: tert-Amyl alcohol / 70 °C / Resolution of racemate; Large scale reaction 4.1: potassium carbonate / water; toluene / 6 h / 50 °C View Scheme |
-
-
64-18-6
formic acid

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

-
-
380636-49-7
(R)-(+)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethylphenol formate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; | 100% |
-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

-
-
380636-50-0
(R)-5-hydroxymethyl tolterodine fumarate salt

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 0.5h; | 100% |
| In acetone at 25 - 30℃; for 1h; | 2.4 g |
-
-
79-30-1
isobutyryl chloride

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

-
-
286930-02-7
fesoterodine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 20℃; Product distribution / selectivity; Inert atmosphere; | 98% |
| With sodium hydroxide In water; toluene at 20℃; for 0.166667h; Product distribution / selectivity; Inert atmosphere; | 98% |
| With sodium hydroxide In water; toluene at 20℃; Inert atmosphere; | 98% |
-
-
79-30-1
isobutyryl chloride

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 20℃; for 0.166667h; Inert atmosphere; | 98% |
-
-
79-30-1
isobutyryl chloride

-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: isobutyryl chloride; (R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol With triethylamine In dichloromethane at 0 - 30℃; for 0.75h; Stage #2: (2E)-but-2-enedioic acid In cyclohexane; butanone at 0 - 20℃; | 71% |

-
-
79-30-1
isobutyryl chloride

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

-
A
-
286930-02-7
fesoterodine

-
B
-
1208313-13-6
C30H43NO4

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -10 - -5℃; for 2.5h; Product distribution / selectivity; | |
| With triethylamine In dichloromethane at -10 - -5℃; for 2.5h; Product distribution / selectivity; | |
| Stage #1: isobutyryl chloride; (R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol In dichloromethane at -10 - -5℃; for 2.5h; Stage #2: With sodium carbonate In dichloromethane; water Product distribution / selectivity; |

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol
-
-
17199-29-0
(S)-Mandelic acid

-
-
79-30-1
isobutyryl chloride

-
-
207679-81-0
(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol

-
-
1206695-46-6
2-[(1R)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-hydroxy-methylphenyl isobutyrate mandelate

| Conditions | Yield |
|---|---|
| Stage #1: isobutyryl chloride; (R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)-phenol In dichloromethane at -10 - 0℃; Stage #2: With sodium hydrogencarbonate In dichloromethane; water for 0.25h; Stage #3: (S)-Mandelic acid In isopropyl alcohol at 10 - 55℃; for 7.5h; |

Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View