-
Name
(-)-1-[(4-Chlorophenyl)phenylmethyl]piperazine
- EINECS
- CAS No. 300543-56-0
- Article Data10
- CAS DataBase
- Density 1.158
- Solubility Soluble in chloroform, methanol, and dichloromethane. Slightly soluble in water.
- Melting Point 91-93oC
- Formula C17H19 Cl N2
- Boiling Point 409.1oC at 760 mmHg
- Molecular Weight 286.804
- Flash Point 193.3oC
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (-)-1-[(4-Chlorophenyl)phenylmethyl]piperazine;(R)-1-(p-Chlorobenzhydryl)piperazine
- PSA 15.27000
- LogP 3.60130
Synthetic route
-
-
1161573-31-4
(R)-(-)-1-[(4-chlorophenyl)phenylmethyl]piperazine N-acetyl-L-phenylalanine salt

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water at 20℃; for 12.5h; Product distribution / selectivity; | 99% |
| With sodium hydroxide; water In ethyl acetate Product distribution / selectivity; | 90% |
-
-
943987-59-5
(-)-1-[(4-chlorophenyl)phenylmethyl]-4-[(4-methoxyphenyl)sulfonyl]piperazine

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: (-)-1-[(4-chlorophenyl)phenylmethyl]-4-[(4-methoxyphenyl)sulfonyl]piperazine With hydrogen bromide; acetic acid at 70 - 75℃; for 3h; Industry scale; Stage #2: With sodium hydroxide In water at 0 - 35℃; pH=~ 12 - ~ 14; Product distribution / selectivity; Industry scale; | 92.7% |
-
-
942283-97-8
(-)-1-[(4-chlorophenyl)phenylmethyl]-4-[(4-methylphenyl)sulfonyl]piperazine

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: (-)-1-[(4-chlorophenyl)phenylmethyl]-4-[(4-methylphenyl)sulfonyl]piperazine With hydrogen bromide; acetic acid; 4-hydroxy-benzoic acid at 25℃; for 17h; Stage #2: With sodium hydroxide In water Product distribution / selectivity; | 84.8% |
-
-
821-48-7
bis-(2-chloroethyl)amine hydrochloride

-
-
163837-57-8
(R)-(4-chlorophenyl)(phenyl)methylamine

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: bis-(2-chloroethyl)amine hydrochloride; (R)-(4-chlorophenyl)(phenyl)methylamine With N-ethyl-N,N-diisopropylamine for 3h; Heating / reflux; Stage #2: With diethylamine at 60℃; for 5h; Heating / reflux; Stage #3: With sodium hydroxide; water pH=10 - 11; | |
| Stage #1: bis-(2-chloroethyl)amine hydrochloride; (R)-(4-chlorophenyl)(phenyl)methylamine With N-ethyl-N,N-diisopropylamine for 3h; Reflux; Stage #2: With diethylamine at 60℃; Reflux; |
-
-
941576-99-4
(R)-(+)-4-(4-chlorophenyl)-phenylmethyl-piperazine-1-carboxylic acid-2,2,2-trichloroethylester

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol at 20 - 100℃; Product distribution / selectivity; |
-
-
1136010-90-6
C24H23ClN2O2

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol for 3 - 3.5h; Product distribution / selectivity; Heating / reflux; | n/a |
-
-
1155402-57-5
C25H25ClN2O2

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol for 1.5h; Product distribution / selectivity; Heating / reflux; |
-
-
1155402-66-6
C25H25ClN2O3

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol for 2.75h; Product distribution / selectivity; Heating / reflux; |
-
-
1155402-68-8
C24H22ClN3O4

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol at 50℃; for 3.5h; Product distribution / selectivity; |
-
-
1136010-92-8
C25H25ClN2O2

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In isopropyl alcohol at 100℃; for 5h; Product distribution / selectivity; |
-
-
1092460-01-9
(-)-1-[(4-chlorophenyl)phenylmethyl]-4-[(phenyl)sulfonyl]piperazine

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: (-)-1-[(4-chlorophenyl)phenylmethyl]-4-[(phenyl)sulfonyl]piperazine With hydrogen bromide; acetic acid at 25 - 60℃; for 5h; Stage #2: With water; sodium hydroxide In toluene |
-
-
1228582-98-6
C28H37ClN2O2*H2O4S

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: C28H37ClN2O2*H2O4S With sulfuric acid In isopropyl alcohol at 20 - 90℃; Stage #2: With sodium hydroxide In di-isopropyl ether; water; isopropyl alcohol |

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: C28H37ClN2O2*(x)H2O4S With sulfuric acid; isopropyl alcohol at 20 - 90℃; Stage #2: With sodium hydroxide In water pH=8; |
-
-
163837-57-8
(R)-(4-chlorophenyl)(phenyl)methylamine

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine / 128 - 130 °C 2: potassium hydroxide / isopropyl alcohol / 9 h / 80 - 85 °C View Scheme |

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 65 - 70℃; for 0.5h; |
-
-
1391851-21-0
C22H27ClN2O2

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In isopropyl alcohol at 80 - 85℃; for 9h; |

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid In dichloromethane at 10℃; for 3h; Inert atmosphere; |
-
-
31250-08-5
2-chloroethyl cyanomethyl ether

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloroethyl cyanomethyl ether; (R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine With sodium carbonate; potassium iodide In acetonitrile at 110 - 115℃; for 20h; Stage #2: With hydrogenchloride In acetonitrile for 0.333333h; pH=0.5 - 1; | 95% |
-
-
107-07-3
2-chloro-ethanol

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
705289-61-8
(+)-[2-[4-[(4-chlorophenyl)-phenyl methyl]-1-piperazinyl]]ethanol

| Conditions | Yield |
|---|---|
| With triethylamine at 80 - 105℃; for 5.5 - 6h; Industry scale; | 90.49% |
| With sodium carbonate; potassium iodide In toluene for 24h; Reflux; |
-
-
14869-41-1
(2-chloroethoxy)-acetic acid

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
130018-77-8
levocetirizine

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydride In N,N-dimethyl-formamide at 95℃; for 5h; Inert atmosphere; | 86.5% |
-
-
109-70-6
1,3-chlorobromopropane

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
942132-43-6
C20H24Cl2N2

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 55℃; | 70% |

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine


| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In N,N-dimethyl-formamide; toluene Heating; | A 36% B n/a |


| Conditions | Yield |
|---|---|
| In ethanol at 5 - 20℃; Product distribution / selectivity; | 16.2% |
| In ethanol; ethyl acetate at 5 - 20℃; Product distribution / selectivity; | n/a |
| In ethanol; ethyl acetate at 5 - 20℃; Product distribution / selectivity; | n/a |
| In isopropyl alcohol at 80 - 85℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol |

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol |

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol |

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine


| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In N,N-dimethyl-formamide; toluene Heating; |

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
299461-25-9
4-[4-(2-Bromoethoxy)phenyl]but-3-yn-1-ol

-
-
299461-26-0
4-[4-(2-{4-[(R)-(4-Chloro-phenyl)-phenyl-methyl]-piperazin-1-yl}-ethoxy)-phenyl]-but-3-yn-1-ol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
802982-14-5
4-[4-(3-bromo-propoxy)-phenyl]-but-3-yn-1-ol

-
-
299461-37-3
4-[4-(3-{4-[(R)-(4-Chloro-phenyl)-phenyl-methyl]-piperazin-1-yl}-propoxy)-phenyl]-but-3-yn-1-ol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
299461-39-5
4-[4-(4-bromo-butoxy)-phenyl]-but-3-yn-1-ol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
4480-83-5
1,4-dioxane-2,6-dione

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
1058165-14-2
R-2-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)-2-oxoethoxy)acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dioxane-2,6-dione; (R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine In acetonitrile for 12h; Heating / reflux; Stage #2: With sodium hydroxide; water In dichloromethane for 0.333333h; Stage #3: In dichloromethane; water pH=4 - 4.5; Product distribution / selectivity; Acidic conditions; | |
| Stage #1: 1,4-dioxane-2,6-dione; (R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine With tetrabutylammomium bromide In dimethyl sulfoxide for 10h; Stage #2: With sodium hydroxide; water In Isopropyl acetate pH=10; Stage #3: In Isopropyl acetate; water pH=4 - 4.5; Product distribution / selectivity; Acidic conditions; | |
| With tetrabutylammomium bromide In dimethyl sulfoxide for 10h; Product distribution / selectivity; |
-
-
107-04-0
1-Bromo-2-chloroethane

-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 55℃; |
-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
1159975-50-4
C28H30ClN5O2S2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / toluene / 55 °C 2: potassium carbonate; triethylamine / acetone / Reflux View Scheme |
-
-
300543-56-0
(R)-1-((4-chlorophenyl)(phenyl)methyl)piperazine

-
-
1159975-51-5
C31H35ClN6O2S

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / toluene / 55 °C 2: potassium carbonate; triethylamine / acetone / Reflux View Scheme |
(-)-1-[(4-Chlorophenyl)phenylmethyl]piperazine Chemical Properties
Systematic Name: 1-[(4-Chlorophenyl)(phenyl)methyl]piperazine
Synonyms of (-)-1-[(4-Chlorophenyl)phenylmethyl]piperazine (CAS NO.300543-56-0): (R)-1-(p-Chlorobenzhydryl)piperazine
CAS NO: 300543-56-0
Molecular Formula: C17H19ClN2
Molecular Weight: 286.80
Molecular Structure: 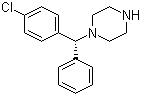
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 3
Polar Surface Area: 6.48Å2
Index of Refraction: 1.592
Molar Refractivity: 83.82 cm3
Molar Volume: 247.6 cm3
Surface Tension: 44 dyne/cm
Density: 1.158 g/cm3
Flash Point: 193.3 °C
Enthalpy of Vaporization: 66.12 kJ/mol
Boiling Point: 409.1 °C at 760 mmHg
Vapour Pressure: 6.66E-07 mmHg at 25°C
SMILES: Clc1ccc(cc1)C(c2ccccc2)N3CCNCC3
InChI: InChI=1/C17H19ClN2/c18-16-8-6-15(7-9-16)17(14-4-2-1-3-5-14)20-12-10-19-11-13-20/h1-9,17,19H,10-13H2
InChIKey: UZKBSZSTDQSMDR-UHFFFAOYAS
Std. InChI: InChI=1S/C17H19ClN2/c18-16-8-6-15(7-9-16)17(14-4-2-1-3-5-14)20-12-10-19-11-13-20/h1-9,17,19H,10-13H2
Std. InChIKey: UZKBSZSTDQSMDR-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View