-
Name
PERFLUORO-TERT-BUTANOL
- EINECS
- CAS No. 2378-02-1
- Article Data26
- CAS DataBase
- Density 1.67g/cm3
- Solubility
- Melting Point -17°C
- Formula C4H F9 O
-
Boiling Point
45 °C(lit.)
- Molecular Weight 236.037
- Flash Point 5.1°C
- Transport Information
- Appearance clear colorless liquid
- Safety Low toxicity by inhalation. When heated to decomposition it emits toxic vapors of F−.
- Risk Codes 20-36/37/38-23
-
Molecular Structure
- Hazard Symbols Xn,Xi,T
- Synonyms 1,1,1,3,3,3-Hexafluoro-2-(trifluoromethyl)-2-propanol;2,2,2-Trifluoro-1,1-bis(trifluoromethyl)ethanol; 2-(Trifluoromethyl)-1,1,1,3,3,3-hexafluoro-2-propanol;Nonafluoro-tert-butanol; Nonafluoro-tert-butyl alcohol; Perfluoro tert-butylalcohol; Perfluoro(2-methyl-2-propanol); Perfluoro-tert-butanol;Perfluoro-tert-butyl alcohol; TBOL; Tris(trifluoromethyl)methanol
- PSA 20.23000
- LogP 2.40440
Synthetic route
-
-
7440-69-9
bismuth

-
-
27579-40-4
tris-(trifluoromethyl)methyl hypochlorite

-
A
-
88392-03-4
Bi(3+)*3(CF3)3CO(1-) = Bi{(CF3)3CO}3

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| byproducts: (CF3)2CO, CF3Cl, Cl2; reaction mixt. maintained at 0°C for 40 h and at room temp. for 94 h and between 25 and 30°C for 6 h; addn. of additional (CF3)3COCl; volatiles removed at room temp. under vac.; elem. anal.; | A 93% B n/a |

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
684-16-2
Hexafluoroacetone

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With potassium fluoride In acetonitrile Mechanism; a) 9 h, -40 deg C, b) 3 h, 18 deg C; other carbonyl compounds, variation of conditions: xfluorinated tertiary alcohols and alkoxides from nucleophilic trifluoromethylation of carbonyl compounds; | 91.3% |
| With potassium fluoride In acetonitrile 1) -40 deg C, 9 h, 2) 18 deg C, 3 h; | 91.3% |
-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
407-25-0
trifluoroacetic anhydride

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With tetramethylammonium fluoride In 1,2-dimethoxyethane at -30 - 20℃; | 87% |
-
-
7594-49-2
1,1,1-trichloro-3,3,3-trifluoro-2-(trifluoromethyl)propan-2-ol

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With antimony pentafluoride for 3h; Heating; | 85% |
| With antimony pentafluoride |
-
-
353-50-4
Carbonyl fluoride

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With potassium fluoride In acetonitrile for 18h; -40 deg C to r.t.; | 77.1% |
-
-
2809-92-9
nanofluoro-tert-butylamine

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; sodium nitrite In water at 20℃; for 1h; | 70% |
-
-
75-38-7
Vinylidene fluoride

-
-
178984-96-8
C4BrF9O

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
684-16-2
Hexafluoroacetone

| Conditions | Yield |
|---|---|
| at -88 - 24℃; for 12h; Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; | A n/a B n/a C 31% D n/a |
| at -88 - 24℃; for 12h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
178984-96-8
C4BrF9O

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
684-16-2
Hexafluoroacetone

| Conditions | Yield |
|---|---|
| at -88 - 24℃; for 12h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |


-
-
1493-13-6
trifluorormethanesulfonic acid

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
71212-59-4
bis(nonafluoro-tert-butoxy)(pentafluorophenylsulfanyl)trifluorophenylsulfur(IV)

-
-
7732-18-5
water

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
1494-06-0
bis(pentafluorophenyl) disulfide

-
C
-
63113-87-1
pentafluorophenyl pentafluorobenzenethiosulfonate

| Conditions | Yield |
|---|---|
| hydrolysis at 25°C; |

-
-
71192-83-1
bis(nonafluoro-tert-butoxy)(trifluoromethyl)trifluoromethylsulfanylsulfur(IV)

-
-
7732-18-5
water

-
A
-
372-64-5
Bis(trifluoromethyl)disulfid

-
B
-
358-15-6
Trifluoro-methanethiosulfonic acid S-trifluoromethyl ester

-
C
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| hydrolysis at 25°C; |

-
-
2809-92-9
nanofluoro-tert-butylamine

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
354-93-8
1,1,1,3,3,3-hexafluoro-2-nitroso-2-trifluoromethyl-propane

| Conditions | Yield |
|---|---|
| With sodium nitrite In sulfuric acid At 0°C then at -10°C; | |
| With NaNO2 In sulfuric acid aq. H2SO4; At 0°C then at -10°C; |
-
-
52225-54-4
nonafluoro-tert-butyl trifluoromethanesulfinate

-
A
-
353-50-4
Carbonyl fluoride

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
C
-
7783-61-1
silicon tetrafluoride

| Conditions | Yield |
|---|---|
| byproducts: SO2; hydrolyzes in moist air at 25°C; |

-
-
88391-99-5
CrO2{(CF3)3CO}2

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
7738-94-5
chromic acid

| Conditions | Yield |
|---|---|
| With water detected by NMR; |
-
-
1192744-27-6
C16H12F9NO4

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With NADPH:cytochrome P450 reductase; NADPH In water-d2; water; acetonitrile at 37℃; for 0.25h; pH=7.6; Kinetics; Concentration; Reagent/catalyst; aq. phosphate buffer; Inert atmosphere; Enzymatic reaction; |

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

-
A
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |
-
-
1086659-07-5
PhF-Al(OC(CF3)3)3

-
-
7732-18-5
water

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
1436826-20-8, 1436826-39-9
C12H2AlF27O4

| Conditions | Yield |
|---|---|
| In fluorobenzene Schlenk technique; |


-
-
75-65-0
tert-butyl alcohol

-
A
-
420-56-4
trimethylsilyl fluoride

-
B
-
107-46-0
Hexamethyldisiloxane

-
C
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In dichloromethane; dichloromethane-d2 at -30 - 20℃; Inert atmosphere; Schlenk technique; Glovebox; |

-
-
18173-64-3
tert-butyldimethylsilanol

-
A
-
107-46-0
Hexamethyldisiloxane

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
C
-
2357-76-8
tert-butyldimethylfluorosilane

| Conditions | Yield |
|---|---|
| In dichloromethane; dichloromethane-d2 at -78℃; Inert atmosphere; Schlenk technique; Glovebox; |

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
2203-56-7
nonafluoro-tert-butyl hypofluorite

| Conditions | Yield |
|---|---|
| With fluorine; cesium fluoride at -196 - 10℃; for 3 - 4h; Inert atmosphere; | 100% |
| With cesium fluoride; fluorine at -78℃; for 0.333333h; | 98% |
| Stage #1: 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol With cesium fluoride at -196 - 20℃; Stage #2: With fluorine at -78℃; for 0.333333h; | 97% |
| With fluorine | |
| With fluorine; cesium fluoride at -78 - 20℃; under 225.023 Torr; |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.5h; | 100% |
| With sodium hydride In diethyl ether at 20℃; for 2h; Inert atmosphere; Glovebox; | 84% |
| With sodium hydride In diethyl ether at 20℃; for 3h; Inert atmosphere; Glovebox; Cooling with ice; | 80% |
| With sodium hydride In diethyl ether |
-
-
16853-85-3
lithium aluminium tetrahydride

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
274933-96-9
Li[AlPFTB4]

| Conditions | Yield |
|---|---|
| In pentane at 20℃; | 100% |
| In diethyl ether; toluene for 21h; Inert atmosphere; Reflux; |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
13100-46-4
1,2,3,4-tetra-O-acetyl-β-D-glucopyranose

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol; 1,2,3,4-tetra-O-acetyl-β-D-glucopyranose With triphenylphosphine In tetrahydrofuran at 20 - 75℃; for 0.0833333h; Inert atmosphere; Stage #2: With di-isopropyl azodicarboxylate In tetrahydrofuran at 75℃; for 3.3h; Inert atmosphere; | 99.9% |
-
-
380907-37-9
Re(C3(OH)(C6H5)2)(CO)2(CH3C(CH2P(C6H5)2)3)

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 under N2, in NMR tube; 10 equiv. of HOC(CF3)3 syringed into soln. of Re complex in CD2Cl2 cooled to -78°C (dry ice-acetone bath); not isolated; detd. by (31)P NMR spectroscopy; | 99% |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
29646-16-0
potassium perfluoro-tert-butoxide

| Conditions | Yield |
|---|---|
| With potassium hydride In diethyl ether at -78 - 20℃; Inert atmosphere; | 99% |
| With potassium hydroxide In ethanol at 0 - 20℃; for 0.5h; Glovebox; | 93% |
| With potassium hydride In diethyl ether Inert atmosphere; Glovebox; | |
| With potassium hydride In diethyl ether |
-
-
7440-69-9
bismuth

-
-
27579-40-4
tris-(trifluoromethyl)methyl hypochlorite

-
A
-
88392-03-4
Bi(3+)*3(CF3)3CO(1-) = Bi{(CF3)3CO}3

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| byproducts: (CF3)2CO, CF3Cl, Cl2; reaction mixt. maintained at 0°C for 40 h and at room temp. for 94 h and between 25 and 30°C for 6 h; addn. of additional (CF3)3COCl; volatiles removed at room temp. under vac.; elem. anal.; | A 93% B n/a |

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
684-16-2
Hexafluoroacetone

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With potassium fluoride In acetonitrile Mechanism; a) 9 h, -40 deg C, b) 3 h, 18 deg C; other carbonyl compounds, variation of conditions: xfluorinated tertiary alcohols and alkoxides from nucleophilic trifluoromethylation of carbonyl compounds; | 91.3% |
| With potassium fluoride In acetonitrile 1) -40 deg C, 9 h, 2) 18 deg C, 3 h; | 91.3% |
-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
407-25-0
trifluoroacetic anhydride

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With tetramethylammonium fluoride In 1,2-dimethoxyethane at -30 - 20℃; | 87% |
-
-
7594-49-2
1,1,1-trichloro-3,3,3-trifluoro-2-(trifluoromethyl)propan-2-ol

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With antimony pentafluoride for 3h; Heating; | 85% |
| With antimony pentafluoride |
-
-
353-50-4
Carbonyl fluoride

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With potassium fluoride In acetonitrile for 18h; -40 deg C to r.t.; | 77.1% |
-
-
2809-92-9
nanofluoro-tert-butylamine

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; sodium nitrite In water at 20℃; for 1h; | 70% |
-
-
75-38-7
Vinylidene fluoride

-
-
178984-96-8
C4BrF9O

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
684-16-2
Hexafluoroacetone

| Conditions | Yield |
|---|---|
| at -88 - 24℃; for 12h; Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; | A n/a B n/a C 31% D n/a |
| at -88 - 24℃; for 12h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
178984-96-8
C4BrF9O

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
684-16-2
Hexafluoroacetone

| Conditions | Yield |
|---|---|
| at -88 - 24℃; for 12h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |


-
-
1493-13-6
trifluorormethanesulfonic acid

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
71212-59-4
bis(nonafluoro-tert-butoxy)(pentafluorophenylsulfanyl)trifluorophenylsulfur(IV)

-
-
7732-18-5
water

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
1494-06-0
bis(pentafluorophenyl) disulfide

-
C
-
63113-87-1
pentafluorophenyl pentafluorobenzenethiosulfonate

| Conditions | Yield |
|---|---|
| hydrolysis at 25°C; |

-
-
71192-83-1
bis(nonafluoro-tert-butoxy)(trifluoromethyl)trifluoromethylsulfanylsulfur(IV)

-
-
7732-18-5
water

-
A
-
372-64-5
Bis(trifluoromethyl)disulfid

-
B
-
358-15-6
Trifluoro-methanethiosulfonic acid S-trifluoromethyl ester

-
C
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| hydrolysis at 25°C; |

-
-
2809-92-9
nanofluoro-tert-butylamine

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
354-93-8
1,1,1,3,3,3-hexafluoro-2-nitroso-2-trifluoromethyl-propane

| Conditions | Yield |
|---|---|
| With sodium nitrite In sulfuric acid At 0°C then at -10°C; | |
| With NaNO2 In sulfuric acid aq. H2SO4; At 0°C then at -10°C; |
-
-
52225-54-4
nonafluoro-tert-butyl trifluoromethanesulfinate

-
A
-
353-50-4
Carbonyl fluoride

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
C
-
7783-61-1
silicon tetrafluoride

| Conditions | Yield |
|---|---|
| byproducts: SO2; hydrolyzes in moist air at 25°C; |

-
-
88391-99-5
CrO2{(CF3)3CO}2

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
7738-94-5
chromic acid

| Conditions | Yield |
|---|---|
| With water detected by NMR; |
-
-
1192744-27-6
C16H12F9NO4

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With NADPH:cytochrome P450 reductase; NADPH In water-d2; water; acetonitrile at 37℃; for 0.25h; pH=7.6; Kinetics; Concentration; Reagent/catalyst; aq. phosphate buffer; Inert atmosphere; Enzymatic reaction; |

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |

-
A
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In octanol at 24.84℃; Equilibrium constant; |
-
-
1086659-07-5
PhF-Al(OC(CF3)3)3

-
-
7732-18-5
water

-
A
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
B
-
1436826-20-8, 1436826-39-9
C12H2AlF27O4

| Conditions | Yield |
|---|---|
| In fluorobenzene Schlenk technique; |


-
-
75-65-0
tert-butyl alcohol

-
A
-
420-56-4
trimethylsilyl fluoride

-
B
-
107-46-0
Hexamethyldisiloxane

-
C
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In dichloromethane; dichloromethane-d2 at -30 - 20℃; Inert atmosphere; Schlenk technique; Glovebox; |

-
-
18173-64-3
tert-butyldimethylsilanol

-
A
-
107-46-0
Hexamethyldisiloxane

-
B
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
C
-
2357-76-8
tert-butyldimethylfluorosilane

| Conditions | Yield |
|---|---|
| In dichloromethane; dichloromethane-d2 at -78℃; Inert atmosphere; Schlenk technique; Glovebox; |

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
2203-56-7
nonafluoro-tert-butyl hypofluorite

| Conditions | Yield |
|---|---|
| With fluorine; cesium fluoride at -196 - 10℃; for 3 - 4h; Inert atmosphere; | 100% |
| With cesium fluoride; fluorine at -78℃; for 0.333333h; | 98% |
| Stage #1: 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol With cesium fluoride at -196 - 20℃; Stage #2: With fluorine at -78℃; for 0.333333h; | 97% |
| With fluorine | |
| With fluorine; cesium fluoride at -78 - 20℃; under 225.023 Torr; |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.5h; | 100% |
| With sodium hydride In diethyl ether at 20℃; for 2h; Inert atmosphere; Glovebox; | 84% |
| With sodium hydride In diethyl ether at 20℃; for 3h; Inert atmosphere; Glovebox; Cooling with ice; | 80% |
| With sodium hydride In diethyl ether |
-
-
16853-85-3
lithium aluminium tetrahydride

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
274933-96-9
Li[AlPFTB4]

| Conditions | Yield |
|---|---|
| In pentane at 20℃; | 100% |
| In diethyl ether; toluene for 21h; Inert atmosphere; Reflux; |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
13100-46-4
1,2,3,4-tetra-O-acetyl-β-D-glucopyranose

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol; 1,2,3,4-tetra-O-acetyl-β-D-glucopyranose With triphenylphosphine In tetrahydrofuran at 20 - 75℃; for 0.0833333h; Inert atmosphere; Stage #2: With di-isopropyl azodicarboxylate In tetrahydrofuran at 75℃; for 3.3h; Inert atmosphere; | 99.9% |
-
-
380907-37-9
Re(C3(OH)(C6H5)2)(CO)2(CH3C(CH2P(C6H5)2)3)

-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 under N2, in NMR tube; 10 equiv. of HOC(CF3)3 syringed into soln. of Re complex in CD2Cl2 cooled to -78°C (dry ice-acetone bath); not isolated; detd. by (31)P NMR spectroscopy; | 99% |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

-
-
29646-16-0
potassium perfluoro-tert-butoxide

| Conditions | Yield |
|---|---|
| With potassium hydride In diethyl ether at -78 - 20℃; Inert atmosphere; | 99% |
| With potassium hydroxide In ethanol at 0 - 20℃; for 0.5h; Glovebox; | 93% |
| With potassium hydride In diethyl ether Inert atmosphere; Glovebox; | |
| With potassium hydride In diethyl ether |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In hexane at -30 - 20℃; | 99% |
-
-
2378-02-1
1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In hexane at -30 - 20℃; | 99% |
1,1,1,3,3,3-Hexafluoro-2-(trifluoro-methyl)-2-propanol Chemical Properties
IUPAC Name: 1,1,1,3,3,3-Hexafluoro-2-(trifluoromethyl)propan-2-ol
The MF of Perfluoro-tert-butanol (CAS NO.2378-02-1) is C4HF9O.
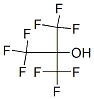
The MW of Perfluoro-tert-butanol (CAS NO.2378-02-1) is 236.04.
Synonyms of Perfluoro-tert-butanol (CAS NO.2378-02-1): 2-Propanol, 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)- ; Nonafluoro-tert-butanol ; Perfluoro-tert-butanol
Apperance: clear colorless liquid
Index of Refraction: 1.263
EINECS: 219-157-3
Density: 1.67 g/ml
Flash Point: 5.1 °C
Boiling Point: 84.7 °C
Melting Point: -17 °C
Storage temp: Keep Cold
BRN: 1841640
1,1,1,3,3,3-Hexafluoro-2-(trifluoro-methyl)-2-propanol Uses
Perfluoro-tert-butanol (CAS NO.2378-02-1) is used as chemical reagent, organic intermediates, fine chemicals, pharmaceutical research and development.
1,1,1,3,3,3-Hexafluoro-2-(trifluoro-methyl)-2-propanol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 10230mg/m3/2H (10230mg/m3) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 521, 1986. |
1,1,1,3,3,3-Hexafluoro-2-(trifluoro-methyl)-2-propanol Safety Profile
Low toxicity by inhalation. When heated to decomposition it emits toxic vapors of F−.Safety information of Perfluoro-tert-butanol (CAS NO.2378-02-1):
Hazard Codes  Xn,Xi,
Xn,Xi, T
T
Risk Statements
20 Harmful by inhalation
36/37/38 Irritating to eyes, respiratory system and skin
23 Toxic by inhalation
Safety Statements
26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
45 In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37 Wear suitable protective clothing and gloves
RIDADR UN 2810 6.1/PG 2
WGK Germany 3
RTECS UB6452700
Hazard Note Toxic
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 23783-26-8
- 23783-42-8
- 23784-96-5
- 23785-19-5
- 23785-21-9
- 23785-22-0
- 23786-13-2
- 23786-14-3
- 23787-80-6
- 23787-90-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View