-
Name
1,1-Cyclohexanediacetic acid mono amide
- EINECS 449-430-4
- CAS No. 99189-60-3
- Article Data11
- CAS DataBase
- Density 1.135 g/cm3
- Solubility
- Melting Point 143-146 °C
- Formula C10H17NO3
- Boiling Point 443.6 °C at760mmHg
- Molecular Weight 199.25
- Flash Point 222.1 °C
- Transport Information
- Appearance White powder
- Safety 22-24/25
- Risk Codes 61
-
Molecular Structure
- Hazard Symbols T
- Synonyms Cyclohexaneaceticacid, 1-(carbamoylmethyl)- (6CI);1,1-Cyclohexanediacetic acid monoamide;1-(Carboxymethyl)cyclohexane-1-acetamide;1-[(Aminocarbonyl)methyl]cyclohexaneacetic acid;
- PSA 80.39000
- LogP 1.98730
Synthetic route
-
-
1130-32-1
3-azaspiro[5,5]undecane-2,4-dione

-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 50℃; for 6h; Reagent/catalyst; | 93% |
| With sodium hydroxide | |
| Stage #1: 3-azaspiro[5.5]undecane-2,4-dione With sodium hydroxide; water at 95 - 105℃; for 6h; Heating / reflux; Stage #2: With hydrogenchloride In water; isopropyl alcohol at 20 - 40℃; for 1h; pH=6.5; Industry scale; Stage #3: With hydrogenchloride In water; isopropyl alcohol at 35 - 40℃; for 1h; pH=4.0 - 4.5; Industry scale; | |
| Stage #1: 3-azaspiro[5.5]undecane-2,4-dione With sodium hydroxide; water for 1h; Heating / reflux; Stage #2: With hydrogenchloride In water at 25℃; pH=5; |
-
-
90961-78-7
spiro[cyclohexane-1,9’-(3,7-diazabicycle-[3.3.1]nonane)]-2’,4’,6’,8’-tetraone

-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 100 - 105℃; for 18h; | 80.8% |
-
-
1010-26-0
1,1-cyclohexanediacetic acid anhydride

-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,1-cyclohexanediacetic acid anhydride With ammonia In water; acetic acid; toluene at 10 - 30℃; for 1.33333h; pH=> 8; Stage #2: With hydrogenchloride In water at 40 - 45℃; for 0.333333h; pH=3.8 - 4.2; | |
| Stage #1: 1,1-cyclohexanediacetic acid anhydride With sodium hydroxide; water; ammonium chloride at 0 - 30℃; for 4h; Stage #2: With hydrogenchloride; water pH=2 - 3; Product distribution / selectivity; | |
| Stage #1: 1,1-cyclohexanediacetic acid anhydride With ammonia; water In isopropyl alcohol at 5℃; for 1h; Stage #2: With hydrogenchloride; water In isopropyl alcohol at 15 - 20℃; for 15h; pH=2 - 3; Product distribution / selectivity; |
-
-
4355-11-7
1,1-cyclohexanediacetic acid

-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: acetic anhydride / 2 h / 130 °C 2.1: ammonia / benzene / 5 h / 25 - 105 °C 2.2: 1.5 h / 55 °C / pH 4 View Scheme | |
| Multi-step reaction with 2 steps 1: acetic anhydride / 0.5 h / 110 °C 2: ammonium hydroxide / 1 h View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
64744-50-9
gabapentin-lactam

| Conditions | Yield |
|---|---|
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hydroxide; sodium hypochlorite In water at 0 - 40℃; for 3.5h; Stage #2: With hydrogenchloride In water at 100 - 105℃; for 3h; pH=8.2 - 8.8; Product distribution / selectivity; Heating / reflux; | 100% |
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hydroxide; sodium hypochlorite In water at 0 - 50℃; for 3h; Stage #2: With hydrogenchloride In water at 100 - 105℃; for 3h; pH=11 - 12; Product distribution / selectivity; | 93.8% |
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hydroxide In water at 20 - 30℃; for 0.5h; Stage #2: With sode de l'acide trichloroisocyanurique In water at 0 - 20℃; for 5h; Stage #3: In water; toluene at 80℃; for 8h; Reagent/catalyst; | 85% |
| Multi-step reaction with 2 steps 1.1: trichloroisocyanuric acid / methanol / 1.75 h / 20 - 25 °C 2.1: sodium hydroxide / water / 4 h / 0 - 20 °C 2.2: 8 h / 80 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In methanol at 20 - 25℃; for 1.75h; | 100% |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
63562-00-5
1-(aminomethyl)-1-cyclohexaneacetic acid sodium salt

| Conditions | Yield |
|---|---|
| With sodium hypobromide; sodium hydroxide In water at 40 - 55℃; Reagent/catalyst; Temperature; Hofmann Rearrangement; Flow reactor; | 91.98% |
| With sodium hydroxide; sodium hypobromide In water at 0 - 10℃; for 2 - 3h; Product distribution / selectivity; Hofmann Rearrangement; | 67% |
| With sodium hydroxide; sodium hypochlorite In water at 0 - 10℃; for 2 - 3h; Product distribution / selectivity; Hofmann Rearrangement; | 65% |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hydroxide; sodium hypochlorite In water at -5 - 25℃; for 9h; Stage #2: With hydrogenchloride In water; butan-1-ol pH=1.5; | 75% |
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hydroxide; bromine In water at -8 - 40℃; for 2h; Stage #2: With hydrogenchloride; water for 4h; pH=2; | |
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / water / 0.5 h / 20 - 30 °C 1.2: 5 h / 0 - 20 °C 1.3: 8 h / 80 °C 2.1: hydrogenchloride; water / 10 h / 100 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: trichloroisocyanuric acid / methanol / 1.75 h / 20 - 25 °C 2.1: sodium hydroxide / water / 4 h / 0 - 20 °C 2.2: 8 h / 80 °C 3.1: hydrogenchloride; water / 10 h / 100 °C View Scheme | |
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hypochlorite; sodium hydroxide In water; benzene at 100℃; Stage #2: at 95℃; for 3h; Temperature; |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hydroxide; sodium hypochlorite In water at -5 - 25℃; for 9h; Stage #2: With sulfuric acid In water; butan-1-ol pH=1.5; | 73.8% |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
60142-96-3
H-Gpn-OH

| Conditions | Yield |
|---|---|
| With sodium carbonate In benzyl alcohol pH=7 - 7.5; Product distribution / selectivity; | 68.6% |
| With sodium hydroxide; sodium hypochlorite In water at -10 - 20℃; for 8h; Hofmann Rearrangement; | |
| Stage #1: [1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid With sodium hypochlorite; sodium hydroxide In water at 40℃; Hofmann Rearrangement; Stage #2: With sodium metabisulfite In water Product distribution / selectivity; |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-02-4
2-(1-((methyleneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-03-5
2-(1-((ethylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-04-6
2-(1-((benzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-05-7
2-(1-((4-methoxybenzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-06-8
2-(1-((2-hydroxybenzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-08-0
2-(1-((2-chloro-6-fluorobenzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-09-1
2-(1-((2-chlorobenzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-10-4
2-(1-((4-fluorobenzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-11-5
2-(1-((4-ethoxybenzylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-12-6
2-(1-(((pyridin-2-yl)methyleneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-13-7
2-(1-((1-(4-ethoxyphenyl)ethylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-14-8
2-(1-((1-(pyridin-2-yl)ethylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-15-9
C18H22N2O2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-17-1
2-(1-((1-(pyridin-3-yl)ethylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1262837-18-2
2-(1-((1-(naphthalen-2-yl)ethylideneamino)methyl)cyclohexyl)acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hypobromide; sodium hydroxide / 15 h / 55 °C 2: sulfuric acid / ethanol / 20 °C View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hypochlorite; sodium hydroxide / benzene; water / 100 °C 1.2: 3 h / 95 °C 2.1: 2 h / pH 5 View Scheme |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

| Conditions | Yield |
|---|---|
| With alkali metal hypohalogenate Hofmann Rearrangement; |
-
-
99189-60-3
[1-(2-amino-2-oxoethyl)cyclohexyl]acetic acid

-
-
1121-89-7
piperidine-2,6-dione

| Conditions | Yield |
|---|---|
| With acetic acid In toluene at 70℃; for 6h; Temperature; |
1,1-Cyclohexanediacetic acid mono amide Chemical Properties
Empirical Formula: C10H17NO3
Molecular Weight: 199.2469 g/mol
EINECS: 449-430-4
Index of Refraction: 1.497
Density: 1.135 g/cm3
Flash Point: 222.1 °C
Melting point: 141-146 °C(lit.)
Appearance: White powder
Enthalpy of Vaporization: 76.88 kJ/mol
Boiling Point: 443.6 °C at 760 mmHg
Vapour Pressure: 4.14E-09 mmHg at 25 °C
Structure of 1,1-Cyclohexanediacetic acid mono amide (CAS NO.99189-60-3):
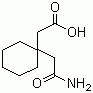
Product Category of 1,1-Cyclohexanediacetic acid mono amide (CAS NO.99189-60-3): Organic acids
1,1-Cyclohexanediacetic acid mono amide Safety Profile
Safety Description of 1,1-Cyclohexanediacetic acid mono amide (CAS NO.99189-60-3): S24/25
S24/25:Avoid contact with skin and eyes.
1,1-Cyclohexanediacetic acid mono amide Specification
1,1-Cyclohexanediacetic acid mono amide , its cas register number is 99189-60-3. It also can be called 3,3-Pentamethyleneglutaramic acid .
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 99199-59-4
- 99199-60-7
- 99199-90-3
- 99200-09-6
- 9920-09-6
- 992-04-1
- 99-20-7
- 99208-50-1
- 99210-65-8
- 99210-90-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View