-
Name
Trimellitic Anhydride
- EINECS 209-008-0
- CAS No. 552-30-7
- Article Data17
- CAS DataBase
- Density 1.669 g/cm3
- Solubility decomposes in water
- Melting Point 163-166 °C(lit.)
- Formula C9H4O5
- Boiling Point 470.6 °C at 760 mmHg
- Molecular Weight 192.128
- Flash Point 201.3 °C
- Transport Information
- Appearance white crystalline powder
- Safety 22-26-36/37/39
- Risk Codes 37-41-42/43
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Trimellitic acid anhydride;1, 3-Dioxo-5-phthalancarboxylic acid;Epon 9150;1,3-Dihydro-1, 3-dioxo-5-isobenzofurancarboxylic acid;4-Carboxyphthalic anhydride;5-Isobenzofurancarboxylic acid,1,3-dihydro-1,3-dioxo-;Trimellitic acid, cyclic 1,2-anhydride;Anhydrotrimellic acid;Trimellic acid anhydride;Trimellic acid 1, 2-anhydride;5-Isobenzofurancarboxylic acid, 1,3-dihydro-1,3-dioxo-;1,3-dioxoisobenzofuran-5-carboxylic acid;1, 2,4-Benzenetricarboxylic acid 1,2-anhydride;Trimellitic acid 1, 2-anhydride;Trimellitic Anhydride(TMA);benzene-1,2,4-tricarboxylic acid 1,2-anhydride;
- PSA 80.67000
- LogP 0.69540
Synthetic route
-
-
13940-95-9
benzene-1,2,4-tricarboxylic acid-2-methyl ester

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| at 200℃; |
-
-
13912-71-5
benzene-1,2,4-tricarboxylic acid-1-methyl ester

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| at 200℃; |
-
-
6051-66-7
2,5-dimethylterephthalic acid

-
A
-
108-31-6
maleic anhydride

-
B
-
89-32-7
Pyromellitic dianhydride

-
C
-
528-44-9
1,2,4-benzene tricarboxylic acid

-
D
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With vanadia at 400℃; Product distribution; effect of temperature on gas-phase oxidation; |

-
-
18190-63-1
2,4-dimethylisophthalic acid

-
A
-
89-32-7
Pyromellitic dianhydride

-
B
-
528-44-9
1,2,4-benzene tricarboxylic acid

-
C
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With vanadia at 400℃; Product distribution; effect of temperature on gas-phase oxidation of methyl-substituted carboxylic acids and anhydrides; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 2: sulfuric acid 3: aqueous-methanolic KOH-solution 5: 200 °C View Scheme | |
| Multi-step reaction with 5 steps 2: sulfuric acid 3: aqueous-methanolic KOH-solution 5: 200 °C View Scheme | |
| Multi-step reaction with 2 steps 1: KMnO4; water; NaOH-solution / man macht schwefelsauer, behandelt mit SO2, saettigt mit Na2SO4 2: 200 - 220 °C / im Vakuum View Scheme | |
| With 1,1,2,2-tetrabromoethane; cobalt(II) acetate; manganese(II) acetate; acetic acid at 220℃; under 15001.5 - 17251.7 Torr; Reagent/catalyst; Temperature; Pressure; Inert atmosphere; Large scale; | 1360 g |
-
-
54699-35-3
1,2-dimethyl 1,2,4-benzenetricarboxylate

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: 200 °C View Scheme | |
| Multi-step reaction with 2 steps 2: 200 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous-methanolic KOH-solution 3: 200 °C View Scheme | |
| Multi-step reaction with 3 steps 1: aqueous-methanolic KOH-solution 3: 200 °C View Scheme |
-
-
22572-84-5
diethyl 4-aminophthalate

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous HCl; aqueous NaNO2 2: aqueous NaOH 3: 180 °C / 0.5 Torr View Scheme |
-
-
105903-40-0
4-cyano-phthalic acid diethyl ester

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous NaOH 2: 180 °C / 0.5 Torr View Scheme |
-
-
243968-20-9
2-amino-5-aminomethyl-4,7-dihydro-5H-thieno[2,3-c]pyran-3-carboxylic acid tert-butyl ester

-
-
552-30-7
trimellitic Anhydride

-
-
330193-56-1
2-(2-amino-3-tert-butoxycarbonyl-4,7-dihydro-5H-thieno[2,3-c]pyran-5-ylmethyl)-1,3-dioxo-2,3-dihydro-1H-isoindole-5-carboxylic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 24h; | 100% |
| In N,N-dimethyl-formamide at 100℃; for 24h; | 100% |
| In N,N-dimethyl-formamide at 100℃; for 24h; | 100% |
-
-
83-07-8
4-amino-2,3-dimethyl-1-phenylpyrazolin-5-one

-
-
552-30-7
trimellitic Anhydride

-
-
292870-53-2
2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1,3-dioxo-2,3-dihydro-1H-isoindole-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide at 20℃; for 72h; | 100% |
| With dmap In N,N-dimethyl-formamide at 110℃; for 12h; | 46% |

| Conditions | Yield |
|---|---|
| In diethylene glycol dimethyl ether at 200℃; for 0.166667h; microwave irradiation; | 99% |
-
-
13822-56-5
3-(trimethoxysilyl)propan-1-amine

-
-
552-30-7
trimellitic Anhydride

-
-
1204776-58-8
C15H21NO8Si

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -5 - 5℃; for 6h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -5 - 5℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 6h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With trimethylamine In tetrahydrofuran byproducts: (CH3)3NHCl; N2-atmosphere; shaking CrCl3 with trace of CrCl2, refluxing with 2 equiv. of phosphinic acid and 2.5 equiv. NMe3 (1 h), cooling to room temp., addn. of 1 equiv. C9H4O5 and excess NMe3, refluxing (0.5 h); filtration, evapn. to dryness, drying (140°C, several days); elem. anal.; | 98% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 20℃; for 12 - 15h; | 98% |

| Conditions | Yield |
|---|---|
| With acetic acid at 130℃; for 16h; Condensation; | 97% |
| In acetic acid for 20h; Reflux; | 95% |
-
-
24313-88-0
3,4,5-Trimethoxyaniline

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With acetic acid at 130℃; for 16h; Condensation; | 97% |

| Conditions | Yield |
|---|---|
| In nitrobenzene at 175 - 180℃; for 3h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(4'-hydroxyphenyl)-2-(4'-aminophenyl)-propane; trimellitic Anhydride In 1-methyl-pyrrolidin-2-one at 20℃; for 6h; Inert atmosphere; Stage #2: In 1-methyl-pyrrolidin-2-one; toluene at 120℃; for 10h; Dean-Stark; | 97% |

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 70 - 180℃; for 9h; Polymerization; | 96% |
-
-
3454-24-8
bis(4-aminophenyl)diphenylsilane

-
-
552-30-7
trimellitic Anhydride

-
-
1357585-01-3
2-(4-((4-(5-carboxy-1,3-dioxoisoindolin-2-yl)phenyl)diphenylsilyl)phenyl)-1,3-dioxoisoindoline-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; for 15h; Reflux; | 96% |

-
-
552-30-7
trimellitic Anhydride

-
-
863990-91-4
2-(4-methoxybenzyloxy)-1,3-dioxoisoindoline-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With pyridine at 90℃; for 24h; | 95% |
-
-
552-30-7
trimellitic Anhydride

-
-
42972-13-4
1,4-dihydroxy-phthalazine-6-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: trimellitic Anhydride With hydrazine In isopropyl alcohol for 5h; Heating / reflux; Stage #2: With hydrogenchloride; water In isopropyl alcohol at 20℃; for 1h; | 95% |
-
-
1374879-15-8
2,6-diamino-4-trifluoromethyl-40-phenoxydiphenyl ether

-
-
552-30-7
trimellitic Anhydride

-
-
1374879-16-9
2,6-bis(N-trimellitimido)-4-trifluoromethyl-40-phenoxydiphenyl ether

| Conditions | Yield |
|---|---|
| With acetic acid for 15h; Inert atmosphere; Reflux; | 95% |
-
-
591-27-5
m-Hydroxyaniline

-
-
552-30-7
trimellitic Anhydride

-
-
82811-05-0
N-(3-hydroxyphenyl)trimellitimide

| Conditions | Yield |
|---|---|
| Stage #1: m-Hydroxyaniline; trimellitic Anhydride In 1-methyl-pyrrolidin-2-one at 20℃; for 6h; Inert atmosphere; Stage #2: In 1-methyl-pyrrolidin-2-one; toluene at 120℃; for 10h; Dean-Stark; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-amino-1-naphthol; trimellitic Anhydride In 1-methyl-pyrrolidin-2-one at 20℃; for 6h; Inert atmosphere; Stage #2: In 1-methyl-pyrrolidin-2-one; toluene at 120℃; for 10h; Dean-Stark; | 95% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 70 - 180℃; for 9h; Polymerization; | 94% |
-
-
38945-21-0
O-allylhydroxylamine hydrochloride

-
-
552-30-7
trimellitic Anhydride

-
-
863990-92-5
2-(allyloxy)-1,3-dioxoisoindoline-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With pyridine at 90℃; for 24h; | 94% |
-
-
123-30-8
4-amino-phenol

-
-
552-30-7
trimellitic Anhydride

-
-
22005-25-0
2-(4-hydroxyphenyl)-1,3-dioxo-2,3-dihydro-1H-isoindole-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-amino-phenol; trimellitic Anhydride In 1-methyl-pyrrolidin-2-one at 20℃; for 6h; Inert atmosphere; Stage #2: In 1-methyl-pyrrolidin-2-one; toluene at 120℃; for 10h; Dean-Stark; | 93% |
| With acetic acid at 130℃; for 16h; Condensation; | 85% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In water at 180 - 208℃; for 3h; Reagent/catalyst; Temperature; Inert atmosphere; | 93% |

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 70 - 180℃; for 9h; Polymerization; | 92% |

| Conditions | Yield |
|---|---|
| With acetic acid at 130℃; for 16h; Condensation; | 92% |
| With acetic acid In diethyl ether Reflux; |

| Conditions | Yield |
|---|---|
| In acetic acid for 10h; Inert atmosphere; Reflux; | 92% |

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With acetic acid at 120℃; for 13h; | 92% |

-
-
552-30-7
trimellitic Anhydride

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 70 - 180℃; for 9h; Polymerization; | 91% |

| Conditions | Yield |
|---|---|
| With acetic acid at 130℃; for 16h; Condensation; | 91% |

| Conditions | Yield |
|---|---|
| With acetic acid at 130℃; for 16h; Condensation; | 91% |
| With acetic acid In diethyl ether Reflux; |
1,2,4-Benzenetricarboxylic anhydride Chemical Properties
Molecular Structure of 1,2,4-Benzenetricarboxylic anhydride (CAS NO.552-30-7):
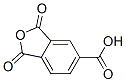
IUPAC Name: 1,3-dioxo-2-benzofuran-5-carboxylic acid
Molecular Formula: C9H4O5
Formula Weight: 192.13
H bond acceptors: 5
H bond donors: 1
Freely Rotating Bonds: 1
Polar Surface Area: 69.67 Å2
Index of Refraction: 1.662
Molar Refractivity: 42.61 cm3
Molar Volume: 115 cm3
Surface Tension: 79.4 dyne/cm
Density: 1.669 g/cm3
Flash Point: 201.3 °C
Enthalpy of Vaporization: 77.24 kJ/mol
Boiling Point: 470.6 °C at 760 mmHg
Melting point: 163-166 °C(lit.)
Vapour Pressure: 1.17E-09 mmHg at 25°C
Sensitive: Moisture Sensitive
EINECS: 209-008-0
Merck: 14,9703
BRN: 9394
Stability: Stable. Moisture sensitive.
InChI
InChI=1/C9H4O5/c10-7(11)4-1-2-5-6(3-4)9(13)14-8(5)12/h1-3H,(H,10,11)
Smiles
c12c(C(=O)OC2=O)ccc(c1)C(O)=O
Product Categories: Industrial/Fine Chemicals; Fluorenes, etc. (reagent for high-performance polymer research); Functional Materials; Reagent for High-Performance Polymer Research
1,2,4-Benzenetricarboxylic anhydride Uses
1,2,4-Benzenetricarboxylic anhydride (CAS NO.552-30-7) is used as an embossing agent for vinyl flooring and as a curing agent for epoxy resins. It is also used as an intermediate for the synthesis of surface coatings chemicals, polymers, dyes printing inks, adhesives, pharmaceuticals . It is mainly used as raw material for polyimide resin, polyester resin, plasticizer TOTM and also be used for producing heatproof & insulating, paint, adhesive,surfactant, synthesize dye materials etc.
1,2,4-Benzenetricarboxylic anhydride Production
1,2,4-trimethylbenzene as raw material, TRIMELLITIC ANHYDRIDE (552-30-7) is synthesized by liquid nitric acid oxidation or cobalt(manganese) catalytic air oxidation.
1,2,4-Benzenetricarboxylic anhydride Toxicity Data With Reference
| 1. | orl-mus LD50:1900 mg/kg | GTPZAB Gigiena Truda i Professionalnye Zabolevaniia. Labor Hygiene and Occupational Diseases. 18 (7)(1974),57. |
1,2,4-Benzenetricarboxylic anhydride Consensus Reports
Reported in EPA TSCA Inventory.
1,2,4-Benzenetricarboxylic anhydride Safety Profile
Moderately toxic by ingestion. Has caused pulmonary edema from inhalation. Irritant to lungs and air passages. May be a powerful allergen. Typical attack consists of breathlessness, wheezing, cough, running nose, immunological sensitization, and asthma symptoms. When heated to decomposition it emits acrid smoke and irritating fumes. See also ANHYDRIDES.
Hazard Codes:  Xn
Xn
Risk Statements: 37-41-42/43
R37:Irritating to respiratory system
R41:Risk of serious damage to the eyes.
R42/43:May cause sensitization by inhalation and skin contact.
Safety Statements: 22-26-36/37/39
S22:Do not breathe dust.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 1
RTECS: DC2050000
F: 10-21
HS Code: 29173980
1,2,4-Benzenetricarboxylic anhydride Standards and Recommendations
OSHA PEL: TWA 0.005 ppm
ACGIH TLV: TWA CL 0.04 mg/m3
DFG MAK: 0.04 mg/m3
NIOSH REL: (Trimellitic Anhydride): handle as extremely toxic
1,2,4-Benzenetricarboxylic anhydride Analytical Methods
For occupational chemical analysis use NIOSH: Trimethyllitic Anhydride, P&CAM 322.
1,2,4-Benzenetricarboxylic anhydride Specification
1,2,4-Benzenetricarboxylic anhydride , with CAS number of 552-30-7, can be called 1,2, 4-Benzenetricarboxylic acid, cyclic 1,2-anhydride ; 5-Phthalanacarboxylic acid, 1,3-dioxo- ; 5-Isobenzofurancarboxylic acid,1,3-dihydro-1,3-dioxo- ; Trimellitic acid, cyclic 1,2-anhydride ; 5-Isobenzofurancarboxylic acid, 1,3-dihydro-1,3-dioxo- . 1,2,4-Benzenetricarboxylic anhydride (CAS NO.552-30-7) is a needle-shaped crystal. Dissolved in water, 2-butanone, acetone, dimethyl formamide and ethyl acetate, cyclohexanone, and soluble in ethanol reacts slightly soluble in carbon tetrachloride, toluene.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 55231-64-6
- 552321-02-5
- 552325-16-3
- 552-32-9
- 552330-86-6
- 552330-87-7
- 552331-00-7
- 552331-05-2
- 552331-06-3
- 552331-15-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View