-
Name
1,2-Dimethoxyethane
- EINECS 203-794-9
- CAS No. 110-71-4
- Article Data84
- CAS DataBase
- Density 0.839 g/cm3
- Solubility Miscible with water
- Melting Point -69 °C
- Formula C4H10O2
- Boiling Point 84.5 °C at 760 mmHg
- Molecular Weight 90.1222
- Flash Point 0 °C
- Transport Information UN 2252 3/PG 2
- Appearance water-white Liquid
- Safety 53-45
- Risk Codes 60-61-11-19-20
-
Molecular Structure
-
Hazard Symbols
 F,
F, T
T
- Synonyms DME (glycol ether);Dimethyl Cellosolve;Ethylene dimethyl ether;Ethylene glycol dimethyl ether;Glycol dimethyl ether;Glyme;Hisolve MMM;Monoethylene glycol dimethyl ether;Monoglyme;a,b-Dimethoxyethane;Ethylene glycol dimethy ether;1,2-Ethanediol, dimethyl ether;2,5-Dioxahexane;
- PSA 18.46000
- LogP 0.27920
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 60℃; | 99.8% |

| Conditions | Yield |
|---|---|
| With MCM-22 In 1,4-dioxane at 50℃; under 750.075 Torr; Pressure; Reagent/catalyst; Temperature; Inert atmosphere; | |
| With hydrogen-type mordenite molecular sieve, Si:Al ratio 4:1 at 200℃; under 60006 Torr; Temperature; Pressure; Reagent/catalyst; Autoclave; |

| Conditions | Yield |
|---|---|
| With sodium Behandeln des Reaktionsgemisches mit Methylchlorid, zuletzt bei 60grad; | |
| With sulfuric acid | |
| With H-type ZSM-5(SiO2/Al2O3=280) at 180℃; for 11h; | |
| With sulfuric acid | |
| With sodium Behandeln des Reaktionsgemisches mit Dimethylsulfat, zuletzt bei 85-90grad; |

| Conditions | Yield |
|---|---|
| With MCM-22 zeolite at 50℃; under 750.075 Torr; Reagent/catalyst; Pressure; Temperature; |

| Conditions | Yield |
|---|---|
| With H-β molecular sieve catalyst at 70℃; under 3750.38 Torr; Reagent/catalyst; Temperature; Pressure; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With NAFION 1100 EW Polymer (H+ form) at 200℃; under 36201.3 - 51716.2 Torr; for 82h; |

-
-
115-10-6
Dimethyl ether

-
-
109-86-4
2-methoxy-ethanol

-
A
-
123-91-1
1,4-dioxane

-
B
-
110-71-4
1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| With sulfonated styrene-divinylbenzene copolymer acidic cation exchange resin (D005) at 130℃; under 37503.8 Torr; Reagent/catalyst; Autoclave; |


| Conditions | Yield |
|---|---|
| In methanol | 73% |
-
-
75-21-8
oxirane

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
6149-41-3
methyl ester (3-hydroxy) propionic acid

-
C
-
107-21-1
ethylene glycol

-
D
-
292638-85-8
acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium tetracarbonylcobaltate at 75℃; under 27752.8 Torr; for 10h; Autoclave; |

-
-
201230-82-2
carbon monoxide

-
A
-
67-56-1
methanol

-
B
-
71-23-8
propan-1-ol

-
C
-
110-71-4
1,2-dimethoxyethane

-
D
-
34557-54-5
methane

-
E
-
64-17-5
ethanol

-
F
-
74-84-0
ethane

-
G
-
78-83-1
2-methyl-propan-1-ol

-
H
-
107-31-3
Methyl formate

-
I
-
75-07-0
acetaldehyde

-
J
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In carbon dioxide; nitrogen at 300℃; under 38787.1 Torr; for 20h; Conversion of starting material; | |
| With hydrogen In carbon dioxide; nitrogen at 275℃; under 38787.1 Torr; for 22h; Conversion of starting material; | |
| With hydrogen In carbon dioxide; nitrogen at 275℃; under 38787.1 Torr; for 22h; Conversion of starting material; | |
| With hydrogen In carbon dioxide; nitrogen at 275℃; under 38787.1 Torr; for 20h; Conversion of starting material; | |
| With hydrogen In carbon dioxide; nitrogen at 275℃; under 38787.1 Torr; for 20h; Conversion of starting material; |


| Conditions | Yield |
|---|---|
| With iron(III) perchlorate In dichloromethane at 20℃; for 1.5h; | 90% |

| Conditions | Yield |
|---|---|
| With alumina at 249.84℃; under 757.576 Torr; Kinetics; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 90℃; for 15h; |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 135℃; Autoclave; |
-
-
115-10-6
Dimethyl ether

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
111-96-6
diethylene glycol dimethyl ether

-
C
-
20637-49-4
1,2,3-trimethoxy glycerol ether

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 150℃; for 4h; Temperature; Autoclave; |

-
-
67-56-1
methanol

-
-
107-21-1
ethylene glycol

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
109-86-4
2-methoxy-ethanol

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide 1.) reflux, 2 h, 2.) a) from 150 deg C to 175 deg C, 5 h, b) from 130 deg C to 160 deg C, 30 min; Yield given. Multistep reaction. Yields of byproduct given; | |
| With NAFION 1100 EW Polymer (H+ form) at 198 - 200℃; under 48096 Torr; for 5h; | |
| With SAPO-34 zeolite In water at 209.84℃; under 2250.23 Torr; for 1.66667h; Catalytic behavior; Reagent/catalyst; Time; Flow reactor; Inert atmosphere; Green chemistry; |

-
-
7440-09-7
potassium

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
92670-64-9
potassium bis({8}annulene)ytterbate(III)

| Conditions | Yield |
|---|---|
| With cyclooctatetraen In ammonia NH3 (liquid); cyclooctatetraene is added to a dry ice/2-propanol cooled liquid NH3 soln. of Yb metal and of K metal;; evapn.;; | A n/a B 86% |

-
-
111-77-3
2-(2-methoxyethoxy)ethyl alcohol

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
112-49-2
Triethylene glycol dimethyl ether

-
C
-
109-86-4
2-methoxy-ethanol

-
D
-
111-96-6
diethylene glycol dimethyl ether

| Conditions | Yield |
|---|---|
| 5%-palladium/activated carbon; nickel at 220℃; under 2250.23 Torr; for 10h; | A 75% B 2% C 10% D 11% |


| Conditions | Yield |
|---|---|
| With water |
-
-
1634-04-4
tert-butyl methyl ether

-
A
-
67-56-1
methanol

-
B
-
110-71-4
1,2-dimethoxyethane

-
C
-
75-28-5
Isobutane

-
D
-
115-11-7
isobutene

| Conditions | Yield |
|---|---|
| HF/Attapulgite at 140 - 150℃; under 1535.79 Torr; for 120 - 384h; Product distribution / selectivity; Gas phase; | |
| mixed metal oxide catalyst (Ce 2.64 wt.percent; Zr 64.12 wt.percent; S 0.69 wt.percent) at 140 - 150℃; under 1535.79 Torr; for 120 - 744h; Product distribution / selectivity; Gas phase; | |
| mixed metal oxide catalyst (W 18.1 wt.percent; Zr 54.3 wt.percent; Fe 0.58 wt.percent; S 0.69 wt.percent) calcined to 500 C in flowing air for 3 h at 140 - 150℃; under 1535.79 Torr; for 24 - 504h; Product distribution / selectivity; Gas phase; |


| Conditions | Yield |
|---|---|
| In water |

-
-
75-21-8
oxirane

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
109-86-4
2-methoxy-ethanol

-
C
-
6149-41-3
methyl ester (3-hydroxy) propionic acid

-
D
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| With immobilization of carbonylcobalt catalyst by poly(4-vinylpyridine)(P4VP) In tetrahydrofuran at 75℃; under 22502.3 Torr; for 15h; | |
| With dicobalt octacarbonyl; triphenylphosphine In tetrahydrofuran at 75℃; under 22502.3 Torr; for 15h; |

-
-
75-21-8
oxirane

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
6149-41-3
methyl ester (3-hydroxy) propionic acid

-
C
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| With immobilization of carbonylcobalt catalyst by poly(4-vinylpyridine)(P4VP) In tetrahydrofuran at 75℃; under 22502.3 Torr; for 15h; |

-
-
75-21-8
oxirane

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
109-86-4
2-methoxy-ethanol

-
C
-
6149-41-3
methyl ester (3-hydroxy) propionic acid

| Conditions | Yield |
|---|---|
| With immobilization of carbonylcobalt catalyst by poly(4-vinylpyridine)(P4VP) In tetrahydrofuran at 75℃; under 22502.3 Torr; for 15h; |


| Conditions | Yield |
|---|---|
| With diethyl ether; copper | |
| With diethyl ether; sodium |
-
-
90669-97-9
bis(bis(trimethylsilyl)amino)ytterbium * 1,2-dimethoxyethane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
110-71-4
1,2-dimethoxyethane

-
C
-
10361-91-8
ytterbium(III) chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In toluene condensing gaseous HCl with liquid nitrogen in an ampul containing toluene solution of (((CH3)3Si)2N)2Yb*CH3O(CH2)2OCH3; warming to room temperature; removing volatile compounds ((CH3)3SiCl, DME) by vac. condensation; vac. sublimation at 100°C (1E-2 mm): NH4Cl;; | A 100% B 90% C 100% D 92% |


| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); poly(phenylsilane) In benzene-d6 at 82 - 85℃; for 3h; sealed; | 96 % Spectr. |

-
A
-
110-71-4
1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| In (2)H8-toluene under N2, dissolution in toluene-d8; not isolated; detected by NMR; |

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
10361-79-2
praseodymium(III) chloride

-
C
-
42371-50-6
tris(pentafuorophenyl)germane

-
D
-
1259-89-8
Chloro[tris(perfluorophenyl)]-germane

| Conditions | Yield |
|---|---|
| With HCl In tetrahydrofuran excess of HCl; in evacuated ampul maintained overnight at 20°C, heated 2 h up to 70°C; organic layer decanted; gas-liq. chromy.; | A 97.5% B 89% C 94.5% D 72.2% E 65.5% |

-
-
3970-21-6
2-Methoxyethoxymethyl chloride

-
A
-
110-71-4
1,2-dimethoxyethane

-
B
-
15112-21-7
bis(chloromethoxy)methane

-
C
-
115-10-6
Dimethyl ether

-
D
-
624-73-7
1,2-Diiodoethane

| Conditions | Yield |
|---|---|
| Stage #1: 2-Methoxyethoxymethyl chloride With niobium(V) iodide In dichloromethane; chloroform-d8 at 20℃; for 48h; Inert atmosphere; Stage #2: With water In dichloromethane; chloroform-d8 Inert atmosphere; |

-
-
110-71-4
1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| With boron trichloride at -78 - 0℃; | 100% |
-
-
110-71-4
1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| In methanol; palladium-carbon | 100% |
| In methanol; palladium-carbon | 100% |
-
-
110-71-4
1,2-dimethoxyethane

-
A
-
90669-97-9
bis(bis(trimethylsilyl)amino)ytterbium * 1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane addn. of a solution of ((CH3)3Si)2N)2Hg in CH3O(CH2)2OCH3 to Yb metal in an evacuated ampul (20°C, 6 h);; decantation, crystn. (pentane), elem. anal.; | A 88% B 100% |

-
-
110-71-4
1,2-dimethoxyethane

-
-
312304-82-8, 827342-25-6
bis(tetrahydrofuran)tris(trimethylsilyl)lutetium(III)

-
-
884911-37-9
Lu(CH2SiMe3)3(THF)(DME)

| Conditions | Yield |
|---|---|
| In pentane all manipulations under dry, oxygen-free N2 atm.; mixt. of DME and pentane added to soln. of Lu compd., slowly cooled to 0°C then to -30°C; white solid pptd., vessel cooled to -78°C, mother liquor decanted, residue dried under vac.; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| With water In 1,2-dimethoxyethane byproducts: (CH3)3SiOSi(CH3)3; DME, (CH3)3SiBr and water added to Pr4O7; suspn. stirred for 8 days at room temp.; dried in vacuum; elem. anal.; | 100% |

-
-
110-71-4
1,2-dimethoxyethane

-
-
4039-32-1
lithium hexamethyldisilazane

-
-
1186017-72-0
[bis(trimethysilyl)amino](pentamethylcyclopentadienyl)[(2-methoxy)ethoxy](methyl)silane

| Conditions | Yield |
|---|---|
| In hexane at -40℃; for 2h; Inert atmosphere; | 100% |


| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane room temp., 10 min; | 100% |

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane room temp., 10 min; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 48h; | 100% |
-
-
110-71-4
1,2-dimethoxyethane

-
-
1384933-47-4
4,5-bis(2,6-diisopropylanilino)-2,7-di-tert-butyl-9,9-dimethylthioxanthene

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane at -78 - 20℃; for 12h; | 100% |
-
-
16940-66-2
sodium tetrahydroborate

-
-
110-71-4
1,2-dimethoxyethane

-
-
55011-46-6
5-nitro-2H-tetrazole

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| at 20℃; for 4h; Inert atmosphere; | A 100% B 3.9 mmol |

-
-
16940-66-2
sodium tetrahydroborate

-
-
110-71-4
1,2-dimethoxyethane

-
-
75393-84-9
5-(trinitromethyl)-2H-tetrazole

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| at 20℃; for 4h; Inert atmosphere; | A 100% B 3.8 mmol |

-
-
16940-66-2
sodium tetrahydroborate

-
-
110-71-4
1,2-dimethoxyethane

-
-
75393-85-0
5-(fluorodinitromethyl)tetrazole

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| at 20℃; for 4h; Inert atmosphere; | A 100% B 3.7 mmol |

-
-
16940-66-2
sodium tetrahydroborate

-
-
110-71-4
1,2-dimethoxyethane

-
-
26621-32-9
3,5-dinitro-1H-1,2,4-triazole

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| at 20℃; for 4h; Inert atmosphere; | A 100% B 4.1 mmol |

-
-
110-71-4
1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| at 5℃; for 168h; Inert atmosphere; Schlenk technique; | 100% |
-
-
110-71-4
1,2-dimethoxyethane

-
-
110-54-3
hexane

-
-
1541157-10-1
[C6H4-1,2-{NC(t-Bu)N(2,6-Me2C6H3)}2]Yb(THF)

-
-
639-58-7
triphenyltin chloride

-
B
-
1064-10-4
hexaphenylditin

| Conditions | Yield |
|---|---|
| Stage #1: [C6H4-1,2-{NC(t-Bu)N(2,6-Me2C6H3)}2]Yb(THF); triphenyltin chloride In tetrahydrofuran for 12h; Schlenk technique; Stage #2: 1,2-dimethoxyethane; hexane Schlenk technique; | A 75% B 100% |

-
-
110-71-4
1,2-dimethoxyethane

-
-
90076-65-6
bis(trifluoromethane)sulfonimide lithium

| Conditions | Yield |
|---|---|
| at 79.84℃; for 0.5h; | 100% |
| With lithium nitrate Concentration; Inert atmosphere; Glovebox; | |
| Glovebox; Sealed tube; Heating; |
-
-
110-71-4
1,2-dimethoxyethane


| Conditions | Yield |
|---|---|
| In toluene for 0.166667h; Inert atmosphere; Schlenk technique; Glovebox; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide; C98H144K3N2O9Ti2(1-)*C24H48KO6(1+) In tetrahydrofuran under 750.075 Torr; for 0.5h; Inert atmosphere; Schlenk technique; Glovebox; Stage #2: 1,2-dimethoxyethane Inert atmosphere; Glovebox; Schlenk technique; | 100% |

-
-
110-71-4
1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate for 12h; | 100% |

| Conditions | Yield |
|---|---|
| for 1h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at -78 - 25℃; for 24h; Inert atmosphere; Schlenk technique; | 100% |

-
-
110-71-4
1,2-dimethoxyethane

-
-
133395-16-1
magnesium bis(trifluoromethane solfonyl)imide

| Conditions | Yield |
|---|---|
| at 100℃; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With H2O In 1,2-dimethoxyethane byproducts: HCl, (CH3)3SiOSi(CH3)3; 4 ds, (Ar); vac. drying, elem. anal.; | 99.8% |


| Conditions | Yield |
|---|---|
| In hexane at 20℃; for 12h; Inert atmosphere; Schlenk technique; | 99.7% |

| Conditions | Yield |
|---|---|
| With water In 1,2-dimethoxyethane byproducts: (CH3)3SiOSi(CH3)3; DME, (CH3)3SiBr and water added to Nd2O3; suspn. stirred for 8 days at room temp.; dried in vacuum; elem. anal.; | 99.6% |

-
-
21205-91-4
9-borabicyclo[3.3.1]nonane dimer

-
-
110-71-4
1,2-dimethoxyethane

-
-
76-05-1
trifluoroacetic acid

-
-
169695-38-9
9-trifluoroacetoxy-9-borabicyclo[3.3.1]nonane, adduct with 1,2-dimethoxyethane

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane byproducts: H2; (inert atmosphere); addn. of CF3COOH to a stirred soln. of the dimer in DME at 0°C, warming to room temp., stirring (14 h); solvent removal (vac.), sublimation; | 99.5% |

1,2-Dimethoxyethane Chemical Properties
Molecular Structure of 1,2-Dimethoxyethane (CAS NO.110-71-4):
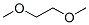
Empirical Formula: C4H10O2
Molecular Weight: 90.121
IUPAC Name: 1,2-dimethoxyethane
H bond acceptors: 2
H bond donors: 0
Freely Rotating Bonds: 3
Polar Surface Area: 18.46 Å2
Index of Refraction: 1.366
Molar Refractivity: 24.07 cm3
Molar Volume: 107.2 cm3
Surface Tension: 21.3 dyne/cm
Density: 0.839 g/cm3
Flash Point: 0 °C
Enthalpy of Vaporization: 32.42 kJ/mol
Boiling Point: 84.5 °C at 760 mmHg
Vapour Pressure: 80.6 mmHg at 25°C
Melting point: -69 °C
Storage temp: 2-8oC
Water Solubility: Miscible
Sensitive: Air Sensitive
Merck: 14,3224
BRN: 1209237
CAS: 110-71-4
InChI
InChI=1/C4H10O2/c1-5-3-4-6-2/h3-4H2,1-2H3
Smiles
C(COC)OC
Classification Code: Reproductive Effect
Stability: Stable, but explosive peroxides may form on exposure to air. Test for the presence of peroxides before heating. Avoid heat, light, air. Highly flammable. Store under inert gas
1,2-Dimethoxyethane Uses
1,2-Dimethoxyethane (CAS NO.110-71-4) is often used as a higher boiling alternative to diethyl ether and THF. As a bidentate ligand, it is used in organometallic chemistry like Grignard reactions, hydride reductions, and palladium-catalyzed reactions like Suzuki reactions and Stille coupling. It is also a good solvent for oligo- and polysaccharides. It is used as the low-viscosity component of the solvent for electrolytes of lithium batteries.
1,2-Dimethoxyethane Production
(1) 2 CH3OCH2CH2OH + 2 Na → 2 CH3OCH2CH2ONa + H2↑
CH3OCH2CH2ONa + CH3Cl → CH3OCH2CH2OCH3 + NaCl
(2) CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3
1,2-Dimethoxyethane Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | National Technical Information Service. Vol. OTS0571323, | |
| mouse | LD50 | oral | 3200mg/kg (3200mg/kg) | National Technical Information Service. Vol. OTS0571323, | |
| rabbit | LDLo | skin | 2gm/kg (2000mg/kg) | National Technical Information Service. Vol. OTS0539769, | |
| rat | LCLo | inhalation | 63gm/m3/6H (63000mg/m3) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: IRRITABILITY | National Technical Information Service. Vol. OTS0571323, |
| rat | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | National Technical Information Service. Vol. OTS0571323, | |
| rat | LDLo | oral | 1gm/kg (1000mg/kg) | National Technical Information Service. Vol. OTS0539769, |
1,2-Dimethoxyethane Consensus Reports
Reported in EPA TSCA Inventory. Glycol ether compounds are on the Community Right-To-Know List.
1,2-Dimethoxyethane Safety Profile
Hazard Codes:  F,
F, T
T
Risk Statements: 60-61-11-19-20
60: May impair fertility
61: May cause harm to the unborn child
11: Highly Flammable
19: May form explosive peroxides
20: Harmful by inhalation
Safety Statements: 53-45
53: Avoid exposure - obtain special instruction before use
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
RIDADR UN: 2252 3/PG 2
WGK Germany: 1
RTECS:KI1451000
F: 3-10-23
HazardClass: 3
PackingGroup: II
HS Code: 29091900
1,2-Dimethoxyethane Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
1,2-Dimethoxyethane Specification
1,2-Dimethoxyethane , with CAS number of 110-71-4, can be called 1,2-Ethanediol, dimethyl ether ; DME ; Dimethyl Cellosolve ; Ethylene dimethyl ether ; Ethylene glycol dimethyl ether ; Glyme ; Monoethylene glycol dimethyl ether ; Monoglyme ; Ethylene glycol dimethy ether ; a,b-Dimethoxyethane . 1,2-Dimethoxyethane (CAS NO.110-71-4) should be stored in a tightly closed container. Make sure to keep from contact with oxidizing materials. It must be stored in a cool, dry, well-ventilated area away from incompatible substances. Flammables-area.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 110714-48-2
- 11071-47-9
- 110720-66-6
- 110-72-5
- 1107-26-2
- 11072-93-8
- 110735-25-6
- 110-73-6
- 11075-20-0
- 11075-30-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View