-
Name
1,1,3,3-TETRAPHENYLDISILOXANE DIOL
- EINECS 213-990-6
- CAS No. 1104-93-4
- Article Data20
- CAS DataBase
- Density 1.21 g/cm3
- Solubility
- Melting Point 113-114 °C
- Formula C24H22O3Si2
- Boiling Point 522.2 °C at 760 mmHg
- Molecular Weight 414.608
- Flash Point 269.6 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Disiloxane-1,3-diol, 1,1,3,3-tetraphenyl-;
- PSA 49.69000
- LogP 1.50080
Synthetic route
-
-
7756-87-8
1,3-dichloro-1,1,3,3-tetraphenyldisiloxane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In tert-Amyl alcohol; toluene hydrolysis at room temp.;; | 92% |
| In tert-Amyl alcohol; toluene hydrolysis at room temp.;; | 92% |
| With water; triethylamine In diethyl ether; acetone at 0 - 20℃; | 81% |
-
-
7647-01-0
hydrogenchloride

-
-
19471-47-7, 15630-86-1, 21545-76-6
cis-dichlorobis(trimethylphosphine)platinum(II)

-
B
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; dichloromethane at 20℃; | A 89% B 64% |


| Conditions | Yield |
|---|---|
| With water at 80℃; Temperature; | 75% |

| Conditions | Yield |
|---|---|
| at 125 - 128℃; | |
| With ammonium hydroxide; acetone | |
| With hydrogenchloride | |
| In not given recrystn. of (C6H5)2Si(OH)2 in presence of acid;; |
-
-
947-42-2
diphenylsilanediol

-
A
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
B
-
1110-85-6
Hexaphenyltrisiloxan-1,5-diol

| Conditions | Yield |
|---|---|
| at 125 - 128℃; |
-
-
18848-24-3
1,3-diethoxy-1,1,3,3-tetraphenyl-disiloxane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide | |
| Multi-step reaction with 2 steps 1: diluted KOH-solution 2: hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether; aqueous dioxane 2: tert-pentyl alcohol; toluene; water View Scheme |
-
-
80-10-4
diphenylsilyl dichloride

-
A
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
B
-
947-42-2
diphenylsilanediol

-
C
-
1110-85-6
Hexaphenyltrisiloxan-1,5-diol

| Conditions | Yield |
|---|---|
| With ammonium carbonate In diethyl ether for 12h; Heating; | A 8.3 g B 8.0 g C n/a |

-
-
947-42-2
diphenylsilanediol

-
-
67-64-1
acetone

-
A
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
B
-
1110-85-6
Hexaphenyltrisiloxan-1,5-diol


-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether; silicon tetrachloride 2: concentrated aqueous ammonia View Scheme | |
| Multi-step reaction with 3 steps 1: diethyl ether; silicon tetrachloride 2: diluted KOH-solution 3: hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether; silicon tetrachloride 2: hydrochloric acid View Scheme |
-
-
80-10-4
diphenylsilyl dichloride

-
A
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
B
-
947-42-2
diphenylsilanediol

| Conditions | Yield |
|---|---|
| With H2O; NH3 In ammonia aq. ammonia=NH3; | |
| With H2O In water |
-
-
947-42-2
diphenylsilanediol

-
-
80-10-4
diphenylsilyl dichloride

-
A
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
B
-
1110-85-6
Hexaphenyltrisiloxan-1,5-diol

| Conditions | Yield |
|---|---|
| mech. sepn. of the crystals;; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: KOH / ethanol 2: not given View Scheme | |
| Multi-step reaction with 2 steps 1: KOH / ethanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: HCl / acetone; chloroform 2: KOH / ethanol View Scheme |

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane at 0 - 20℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; | 100% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane at 0 - 20℃; for 12h; Inert atmosphere; Schlenk technique; Glovebox; | 100% |
-
-
99716-11-7
phenyldimethoxystibine

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: MeOH; Ar; Si-compd. soln. addn. slowly dropwise to Sb-compd. soln. at 0°C, stirring for 30 min at same temp., solvent vac. removal; elem. anal.; | 98% |

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: isopropyl alcohol; soln. of Zr complex in THF added to soln. of Si compound in THF; heated at 50°C for 1h; solvents removed under vac.; the residue dissolved in THF, poured into hexane with vigorous stirring; filtered; elem. anal.; | 96.1% |
-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
54553-25-2
dimethoxymethylstibane

-
-
169828-90-4
2,8-dimethyl-1,3,5,7,9,11-hexaoxa-4,4',6,6',10,10',12,12'-octaphenyl-4,6,10,12-tetrasila-2,8-distibacylododecane

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: MeOH; Ar; Si-compd. soln. addn. slowly dropwise to Sb-compd. soln. at 0°C, stirring for 30 min at same temp., solvent vac. removal; elem. anal.; | 96% |
-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
98-80-6
phenylboronic acid

-
-
136745-52-3
pentaphenylboracyclotrisiloxane

| Conditions | Yield |
|---|---|
| In benzene byproducts: water; refluxing (equimolar mixture, 4 h, continuous removal of water); cooling, filtration, distn. (reduced pressure), recrystn. (Et2O:petroleum ether 1:3); elem. anal.; | 95% |
-
-
37336-24-6
[Cp(CO)2W]2SnCl2

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| With NEt3 In diethyl ether byproducts: [HNEt3]Cl; N2-atmosphere; stirring (room temp., overnight); filtering, evapn. (reduced pressure); elem. anal.; | 95% |
-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
7722-84-1
dihydrogen peroxide

-
-
2001-45-8
tetraphenyl phosphonium chloride

| Conditions | Yield |
|---|---|
| With NaOH In ethanol; water addn. of MoO3 to aq. H2O2, stirring (60°C, 1 h), cooling to room temp., centrifugation, addn. to the siloxane in EtOH, addn. of aq. NaOH,stirring (45 min), pptn. with (PPh4)Cl in EtOH; filtration, washing (H2O; Et2O), drying in air; | 95% |


-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| With NEt3 In diethyl ether byproducts: [HNEt3]Cl; N2-atmosphere; stirring (room temp., overnight), filtering, evapn. (reduced pressure); elem. anal.; | 91% |

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| With NEt3 In diethyl ether byproducts: [HNEt3]Cl; N2-atmosphere; stirring (room temp., overnight); filtering, evapn. (reduced pressure); elem. anal.; | 91% |
-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
1262-21-1
bis(triphenyltin) oxide

-
-
156116-74-4
1,3-bis-(triphenylstannoxy)-1,1,3,3-tetraphenyl-1,3-disiloxane

| Conditions | Yield |
|---|---|
| In benzene byproducts: H2O; refluxing; removing solvent (vac.), recrystn. (60-80 petrol), elem. anal.; | 90% |
-
-
76-09-5
2,3-dimethyl-2,3-butane diol

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
11113-50-1
boric acid

-
-
255849-45-7
O(Si(C6H5)2OB(OC(CH3)2)2)2

| Conditions | Yield |
|---|---|
| In not given byproducts: H2O; inert atmosphere; elem. anal.; | 88.8% |

-
-
1558-25-4
(chloromethyl)trichlorosilane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
A
-
79891-82-0
2-Chloro-2-chloromethyl-4,4,6,6-tetraphenyl-[1,3,5,2,4,6]trioxatrisilinane

| Conditions | Yield |
|---|---|
| With pyridine In toluene for 3h; Heating; | A n/a B 87.6% |

-
-
75-79-6
Methyltrichlorosilane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
100278-00-0
1,1,7,7-tetrachloro-1,7-dimethyl-3,3,5,5-tetraphenyltetrasiloxane

| Conditions | Yield |
|---|---|
| In diethyl ether at -5℃; for 2h; | 87.5% |
-
-
107-41-5
2-methyl-2,4-pentanediol

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
11113-50-1
boric acid

-
-
255849-46-8
O(Si(C6H5)2OBO2C3H3(CH3)3)2

| Conditions | Yield |
|---|---|
| In not given byproducts: H2O; inert atmosphere; elem. anal.; | 87.1% |


-
-
110-71-4
1,2-dimethoxyethane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
1907-33-1
lithium tert-butoxide

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,3,3-tetraphenyldisiloxane-1,3-diol; lithium tert-butoxide In tetrahydrofuran at 20℃; for 3.5h; Inert atmosphere; Stage #2: iron(II) triflate In tetrahydrofuran at 20℃; Inert atmosphere; Stage #3: 1,2-dimethoxyethane In hexane at 20℃; Inert atmosphere; | 87% |

-
-
75-94-5
trichlorovinylsilane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
A
-
56269-45-5
2-Chloro-4,4,6,6-tetraphenyl-2-vinyl-[1,3,5,2,4,6]trioxatrisilinane

| Conditions | Yield |
|---|---|
| With pyridine In toluene for 3h; Heating; | A n/a B 86.4% |

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
5980-97-2
mesitylboronic acid

-
-
144648-00-0
cyclo-1-mesityl-3,3,7,7-tetraphenyl-1-bora-3,5-disiloxane

| Conditions | Yield |
|---|---|
| In benzene byproducts: water; refluxing (equimolar mixture, 4 h, continuous removal of water); cooling, filtration, distn. (reduced pressure), recrystn. (petroleum ether); elem. anal.; | 86% |

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: isopropyl alcohol; soln. of Ti complex in THF added to soln. of Si compound in THF; heated at 50°C for 1h; solvents removed under vac.; the residue dissolved in THF, poured into hexane with vigorous stirring; filtered; elem. anal.; | 85.2% |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieves In tetrachloromethane stirring with molecular sieves overnight; decantation of suspn. from sieves, solvent removal (vac.), trituration with hexane; elem. anal.; | 85% |
-
-
1145663-94-0, 1146220-12-3
(((2,6-Me2NCH2)2C6H3)SbO)2

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In benzene at 20℃; Inert atmosphere; Schlenk technique; | 85% |

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
262446-47-9
[Os3(CO)10(μ-H)(μ-OSiPh2OSiPh2OH)]

| Conditions | Yield |
|---|---|
| In xylene Si-compd.:Os-compd.=2:1, Os-compd. concn. is 1.2E-1 M, under N2, P2O5 asH2O remover, 4 h or 9:1 molar ratio, 1E-1 M Os-compd. concn., and 11 h, resp.; yield were quant. or 50 % from NMR, resp., the first reaction mixt. was evapd. to dryness, residue was chromd. (silica, CH2Cl2/pentane=10:3), elem. anal.; | 84% |
-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
19429-30-2
di-tert-butyltin dichloride

| Conditions | Yield |
|---|---|
| With NEt3 In acetone byproducts: HNEt3Cl; N2-atmosphere; dropwise addn. of Sn-compd. to equimolar amt. of Si-compd. with 2 equiv. NEt3; filtration off of HNEt3Cl, evapn. (vac.), recrystn. (PhMe); elem. anal.; | 84% |
-
-
109-99-9
tetrahydrofuran

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| Stage #1: tetrahydrofuran; n-butyllithium; 1,1,3,3-tetraphenyldisiloxane-1,3-diol In hexane at 0℃; for 5h; Inert atmosphere; Schlenk technique; Glovebox; Stage #2: terbium(III) chloride In hexane Inert atmosphere; Schlenk technique; Glovebox; | 84% |

-
-
109-99-9
tetrahydrofuran

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
1907-33-1
lithium tert-butoxide

| Conditions | Yield |
|---|---|
| Stage #1: tetrahydrofuran; 1,1,3,3-tetraphenyldisiloxane-1,3-diol; lithium tert-butoxide at 20℃; for 3h; Stage #2: chromium(III) chloride tris(tetrahydrofuran) solvate | 84% |

-
-
124-70-9
Dichloromethylvinylsilane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
A
-
1457-02-9
2-Methyl-4,4,6,6-tetraphenyl-2-vinyl-[1,3,5,2,4,6]trioxatrisilinane

-
B
-
109305-36-4
1,7-dichloro-1,7-dimethyl-1,7-divinyl-3,3,5,5-tetraphenyltetrasiloxane

| Conditions | Yield |
|---|---|
| With pyridine In toluene for 3h; Heating; | A n/a B 83.5% |

-
-
95151-58-9
bis(hydroxydiphenylstannyl)methane

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

| Conditions | Yield |
|---|---|
| In toluene heating mixt. of hydroxystannane and hydroxysilane in toluene at 60 °C for 30 min; filtration, solvent evapn. in vac., recrystn. (hexane-CH2Cl2 1:1); elem. anal.; | 81% |
-
-
110-71-4
1,2-dimethoxyethane

-
-
53317-83-2, 14782-78-6
[scandium(III)(chloride)3(tetrahydrofuran)3]

-
-
1104-93-4
1,1,3,3-tetraphenyldisiloxane-1,3-diol

-
-
4039-32-1
lithium hexamethyldisilazane

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane byproducts: LiCl; under N2; LiN(SiMe3)2 added to suspn. of ScCl3(THF)3 in DME; stirred for12 h; treated with solid disiloxanediol; heated under reflux for 1 h; filtered; filtrate concd.; crystd. at 2°C for 1 wk; elem. anal.; | 81% |

1,3-Disiloxanediol,1,1,3,3-tetraphenyl- Specification
The 1,3-Disiloxanediol,1,1,3,3-tetraphenyl-, with the CAS registry number 1104-93-4, is also known as Disiloxane-1,3-diol, 1,1,3,3-tetraphenyl-. This chemical's molecular formula is C24H22O3Si2 and molecular weight is 414.6007. What's more, its systematic name is 1,1,3,3-Tetraphenyldisiloxane-1,3-diol.
Physical properties about 1,3-Disiloxanediol,1,1,3,3-tetraphenyl- are: (1)ACD/LogP: 9.70; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 9.7; (4)ACD/LogD (pH 7.4): 9.7; (5)ACD/BCF (pH 5.5): 1000000; (6)ACD/BCF (pH 7.4): 1000000; (7)ACD/KOC (pH 5.5): 4524315.5; (8)ACD/KOC (pH 7.4): 4522671.5; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 8; (12)Polar Surface Area: 27.69 Å2; (13)Index of Refraction: 1.641; (14)Molar Refractivity: 123.21 cm3; (15)Molar Volume: 341.2 cm3; (16)Polarizability: 48.84×10-24 cm3; (17)Surface Tension: 48.2 dyne/cm; (18)Density: 1.21 g/cm3; (19)Flash Point: 269.6 °C; (20)Enthalpy of Vaporization: 83.75 kJ/mol; (21)Boiling Point: 522.2 °C at 760 mmHg; (22)Vapour Pressure: 9.9E-12 mmHg at 25 °C.
Use of 1,3-Disiloxanediol,1,1,3,3-tetraphenyl-: it is used to produce other chemicals. For example, it is used to produce 1-Chloro-1-phenyltetraphenylcyclotrisiloxane. The reaction occurs with reagent Pyridine and solvent Benzene. The yield is 62%.
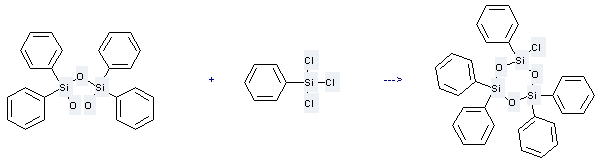
You can still convert the following datas into molecular structure:
(1) SMILES: O([Si](O)(c1ccccc1)c2ccccc2)[Si](O)(c3ccccc3)c4ccccc4
(2) InChI: InChI=1/C24H22O3Si2/c25-28(21-13-5-1-6-14-21,22-15-7-2-8-16-22)27-29(26,23-17-9-3-10-18-23)24-19-11-4-12-20-24/h1-20,25-26H
(3) InChIKey: MYZTUAOLAYIKSJ-UHFFFAOYAT
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 110-49-6
- 110499-66-6
- 110499-97-3
- 1105039-88-0
- 110505-55-0
- 11050-62-7
- 1105067-87-5
- 110-51-0
- 11051-27-7
- 110512-91-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View