-
Name
1,3-THIAZOLIDIN-2-ONE
- EINECS 1312995-182-4
- CAS No. 2682-49-7
- Article Data27
- CAS DataBase
- Density 1.278 g/cm3
- Solubility
- Melting Point 53-54 °C
- Formula C3H5NOS
- Boiling Point 247.6 °C at 760 mmHg
- Molecular Weight 103.145
- Flash Point 103.5 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Oxothiazolidine;NSC 122613;Thiazolidin-2-one;Thiazolidone;2-Thiazolidinone HOMT;1,3-Thiazolan-2-one;
- PSA 54.40000
- LogP 0.77160
Synthetic route

| Conditions | Yield |
|---|---|
| With selenium; oxygen; acetonitrile | 98% |
| Stage #1: carbon monoxide; Cysteamine With potassium carbonate; sulfur In N,N-dimethyl-formamide at 20℃; under 760 Torr; for 4h; Stage #2: With oxygen In N,N-dimethyl-formamide at 20℃; under 760 Torr; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| at 180℃; for 3h; Temperature; Time; Concentration; | 85% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 20℃; for 1h; | 80% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 1h; Sealed tube; | 78% |
-
-
156-57-0
2-mercaptoethylamine hydrochloride

-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-mercaptoethylamine hydrochloride; 1,1'-carbonyldiimidazole With 18-crown-6 ether; potassium carbonate In acetonitrile for 18h; Heating; Stage #2: With water In acetonitrile for 4h; Heating; Further stages.; | 68% |
| In ethanol for 15h; Ambient temperature; | 63% |
| Stage #1: 2-mercaptoethylamine hydrochloride; 1,1'-carbonyldiimidazole With potassium carbonate; 18-crown-6 ether In acetonitrile at 80℃; for 19h; Heating / reflux; Stage #2: With sodium carbonate In water for 1h; Heating / reflux; | 42% |
-
-
134469-06-0
thiazolidine-2-thione

-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With sodium; 2-bromoethanol In ethanol for 4h; Inert atmosphere; Reflux; | 56% |
| With sodium ethanolate; 2-bromoethanol In ethanol at 60℃; for 4h; |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 4h; Inert atmosphere; Reflux; | 56% |
-
-
88512-18-9
(2-mercapto-ethyl)-carbamic acid ethyl ester

-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With hydrotalcite; magnesium aluminum In toluene for 5h; Heating; | 50% |

-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
113511-38-9
S-(2-aminoethyl)-S’-benzyldithiocarbonate hydrochloride

-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With butan-1-ol |

| Conditions | Yield |
|---|---|
| With toluene |
-
-
113511-38-9
S-(2-aminoethyl)-S’-benzyldithiocarbonate hydrochloride

-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With selenium; CO; oxygen In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In diethyl ether; benzene |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 2.5h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile byproducts: H2; Electrochem. Process; electrolyse of a soln. of the ligand in acetonitrile (NH4BF4) with a Nianode at room temp. under N2; filtered, washed with acetonitrile, dried in vac.; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With C29H30F6NOPS In 1,2-dichloro-ethane at 20℃; stereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With C29H30F6NOPS In 1,2-dichloro-ethane at 20℃; stereoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| In acetonitrile byproducts: H2; Electrochem. Process; electrolyse of a soln. of the ligands in acetonitrile (NH4BF4) with a Ni anode at room temp. under N2; filtered, washed with acetonitrile, dried in vac.; elem. anal.; | 96% |


| Conditions | Yield |
|---|---|
| With C29H30F6NOPS In 1,2-dichloro-ethane at 20℃; stereoselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With C29H30F6NOPS In 1,2-dichloro-ethane at 20℃; stereoselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With sodium methansulfinate; tetrabutylammomium bromide; potassium carbonate In tetrahydrofuran at 50℃; for 5h; Green chemistry; regioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With C29H30F6NOPS In 1,2-dichloro-ethane at 20℃; stereoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With C29H30F6NOPS In 1,2-dichloro-ethane at 20℃; stereoselective reaction; | 85% |
-
-
2682-49-7
thiazolidine-2-one

-
-
4897-25-0
5-chloro-1-methyl-4-nitroimidazole

-
-
86230-97-9
3-(3-Methyl-5-nitro-3H-imidazol-4-yl)-thiazolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 15 - 100℃; for 2.25h; | 83% |

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In toluene at 130℃; for 6h; Inert atmosphere; Molecular sieve; | 78% |
-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With 3,3-dimethyl-2-nitroso-2,3-dihydrobenzo[d]isothiazole 1,1-dioxide; trifluoroacetic acid In acetonitrile at 20℃; Sealed tube; | 78% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In acetonitrile for 16h; Heating; | 72% |
| With potassium carbonate; 18-crown-6 ether In acetonitrile for 17h; Heating / reflux; | 55% |
-
-
2682-49-7
thiazolidine-2-one

-
-
15687-27-1
ibuprofen

| Conditions | Yield |
|---|---|
| Stage #1: ibuprofen With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 0.333333h; Stage #2: thiazolidine-2-one In dichloromethane at 20℃; for 4h; | 70% |
-
-
2682-49-7
thiazolidine-2-one

| Conditions | Yield |
|---|---|
| With lithium oxide Microwave irradiation; | 70% |
-
-
2682-49-7
thiazolidine-2-one

-
-
79640-70-3
(S)-5-(tert-butoxy)-4-(4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)-5-oxopentanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (S)-5-(tert-butoxy)-4-(4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)-5-oxopentanoic acid With dmap; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: thiazolidine-2-one In N,N-dimethyl-formamide for 16h; | 65% |
| With dmap; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 16h; | 42% |
-
-
2682-49-7
thiazolidine-2-one

-
-
53207-00-4
1-bromo-2-(bromomethyl)-4,5-dimethoxybenzene

-
-
1018331-29-7
C12H14BrNO3S

| Conditions | Yield |
|---|---|
| Stage #1: thiazolidine-2-one With sodium hydride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 1-bromo-2-(bromomethyl)-4,5-dimethoxybenzene In tetrahydrofuran Reflux; Inert atmosphere; | 63% |
-
-
2682-49-7
thiazolidine-2-one

-
-
175844-54-9
2,3-dimethoxy-6-bromobenzyl bromide

-
-
1018331-30-0
C12H14BrNO3S

| Conditions | Yield |
|---|---|
| Stage #1: thiazolidine-2-one With sodium hydride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 6-bromo-2,3-dimethoxybenzyl bromide In tetrahydrofuran Reflux; Inert atmosphere; | 61% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; acetic acid In acetonitrile at 60℃; for 3h; Electrochemical reaction; Inert atmosphere; | 59% |
-
-
2682-49-7
thiazolidine-2-one

-
-
1567836-15-0
2,2′-(naphthalene-1,4-diylbis(((4-methoxyphenyl)sulfonyl)azanediyl))diacetic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide | 58% |
| Stage #1: 2,2′-(naphthalene-1,4-diylbis(((4-methoxyphenyl)sulfonyl)azanediyl))diacetic acid With dmap; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: thiazolidine-2-one In N,N-dimethyl-formamide | 0.368 g |

| Conditions | Yield |
|---|---|
| With phosphorus(V) oxybromide at 110℃; for 3h; | 57% |

| Conditions | Yield |
|---|---|
| Stage #1: thiazolidine-2-one With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.166667h; Stage #2: 2-(tert-butoxycarbonylamino)ethyl bromide In N,N-dimethyl-formamide at 20℃; for 2h; | 51% |
1,3-Thiazolan-2-one Specification
The 1,3-Thiazolan-2-one, with the CAS registry number 2682-49-7, is also known as Thiazolidin-2-one. It belongs to the product categories of API intermediates; Agricultural chemical; Plant growth modifier; Fosthiazate;fungicide; Pesticide. This chemical's molecular formula is C3H5NOS and molecular weight is 103.14. What's more, its systematic name is 1,3-Thiazolidin-2-one.
Physical properties of 1,3-Thiazolan-2-one are: (1)ACD/LogP: 0.427; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.43; (4)ACD/LogD (pH 7.4): 0.43; (5)ACD/BCF (pH 5.5): 1.24; (6)ACD/BCF (pH 7.4): 1.24; (7)ACD/KOC (pH 5.5): 40.68; (8)ACD/KOC (pH 7.4): 40.68; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 54.4 Å2; (13)Index of Refraction: 1.544; (14)Molar Refractivity: 25.476 cm3; (15)Molar Volume: 80.725 cm3; (16)Polarizability: 10.099×10-24cm3; (17)Surface Tension: 42.7 dyne/cm; (18)Density: 1.278 g/cm3; (19)Flash Point: 103.5 °C; (20)Enthalpy of Vaporization: 56.32 kJ/mol; (21)Boiling Point: 247.6 °C at 760 mmHg; (22)Vapour Pressure: 0.004 mmHg at 25°C.
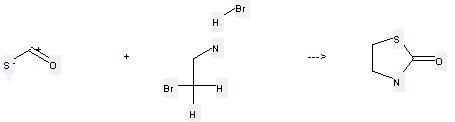
Preparation: this chemical can be prepared by carbon oxide sulfide and 2-bromo-ethylamine; hydrobromide at the temperature of 20 °C. This reaction will need reagent KOH and solvent methanol with the reaction time of 1 hour. The yield is about 80%.
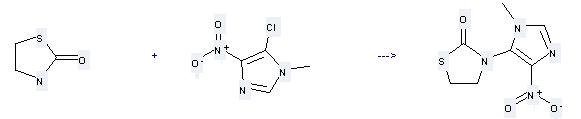
Uses of 1,3-Thiazolan-2-one: it can be used to produce 3-(3-methyl-5-nitro-3H-imidazol-4-yl)-thiazolidin-2-one at the temperature of 15 - 100 °C. It will need reagent NaH and solvent dimethylformamide with the reaction time of 135 min. The yield is about 83%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1SCCN
(2)Std. InChI: InChI=1S/C3H5NOS/c5-3-4-1-2-6-3/h1-2H2,(H,4,5)
(3)Std. InChIKey: SLYRGJDSFOCAAI-UHFFFAOYSA-N
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 26825-89-8
- 26829-43-6
- 26829-47-0
- 26829-89-0
- 26830-30-8
- 26830-40-0
- 26830-95-5
- 26830-96-6
- 26831-44-7
- 26832-08-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View