-
Name
1-Adamantaneacetic acid
- EINECS 225-585-1
- CAS No. 4942-47-6
- Article Data37
- CAS DataBase
- Density 1.167 g/cm3
- Solubility
- Melting Point 134-137 °C(lit.)
- Formula C12H18O2
- Boiling Point 319.2 °C at 760 mmHg
- Molecular Weight 194.274
- Flash Point 163 °C
- Transport Information
- Appearance white to off-white crystalline powder or crystals
- Safety 22-24/25-43-7/8
- Risk Codes 36/37/38-15-10
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn,
Xn,  F
F
- Synonyms Tricyclo[3.3.1.13,7]decane-1-aceticacid;(Adamantan-1-yl)acetic acid;1-(Carboxymethyl)adamantane;NSC 310162;a-(1-Adamantyl)acetic acid;
- PSA 37.30000
- LogP 2.67750
Synthetic route
-
-
104543-11-5
2-(1-adamantyl)-1-nitro-1-(pyridine-thiyl)ethane

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In tetrahydrofuran; methanol at 40℃; for 15h; | 95% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 0 - 2℃; for 2.5h; | 93% |
| (i) H2SO4, BF3, (ii) aq. H2SO4; Multistep reaction; | |
| With sulfuric acid; boron trifluoride |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 93% |
| With potassium hydroxide In methanol Yield given; |
-
-
27174-71-6
methyl 2-(adamantan-1-yl)acetate

-
-
144-55-8
sodium hydrogencarbonate

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide In methanol | 93% |
-
-
144499-21-8
Trifluoro-acetic acid 2-adamantan-1-yl-1-cyano-1-(pyridin-2-ylsulfanyl)-ethyl ester

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In water for 2h; | 91% |
| With potassium carbonate In acetone at 25℃; for 12h; Yield given; |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In water | 87% |
-
-
15782-66-8
ethyl 4-(adamant-1-yl)-phenyl acetate

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol at 20℃; for 1h; | 86% |
-
-
144499-22-9
Phosphoric acid 2-adamantan-1-yl-1-cyano-1-(pyridin-2-ylsulfanyl)-ethyl ester dimethyl ester

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 85% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 5 - 15℃; for 1h; | 80.6% |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: (3r,5r,7r)-1-(2,2-dibromovinyl)adamantane With n-butyllithium In tetrahydrofuran at 0℃; for 1.5h; Stage #2: With 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane In tetrahydrofuran for 2h; | 77% |
-
-
925681-80-7
adamantylacetic acid acid 2-oxo-1,2,2-triphenylethyl ester

-
A
-
201-68-3
13-oxa-indeno[1,2-l]phenanthrene

-
B
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With air In ethanol; acetonitrile Irradiation; | A n/a B 74% |
-
-
27174-71-6
methyl 2-(adamantan-1-yl)acetate

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With bis(tri-n-butyltin)oxide In toluene for 72h; Heating; | 25% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
2094-72-6
1-Adamantanecarbonyl chloride

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With diethyl ether Erwaermen des Reaktionsprodukts mit Ag2O, Na2CO3 und Na2S2O3 in wss. Dioxan; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; boron trifluoride diethyl etherate; tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| (i) BF3, H2SO4, (ii) aq. H2SO4; Multistep reaction; | |
| With sulfuric acid; boron trifluoride |
-
-
16269-13-9
1-Carboxymethyladamantane nitrile

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethylene glycol at 150℃; |
-
-
18220-83-2
adamantan-1-ylacetaldehyde

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate In acetone |
-
-
75-35-4
1,1-Dichloroethylene

-
-
281-23-2
adamantane

-
A
-
17768-28-4
1,3-adamantane diacetic acid

-
B
-
17768-36-4
2-(3-hydroxyadamantan-1-yl)acetic acid

-
C
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid In hexane at 25℃; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With sulfuric acid; nitric acid In hexane at 25℃; Yield given. Yields of byproduct given; | |
| With sulfuric acid; nitric acid In hexane at 25℃; for 4.5h; Product distribution; Mechanism; var. reagent partners concentrations, temp., and solvents; also 1,3-dimethyladamantane; |

-
-
3061-65-2
2-acetoxyacrylonitrile

-
-
828-51-3
1-Adamantanecarboxylic acid

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With 1-hydroxy-2(1H)-pyridinethione; potassium carbonate; dicyclohexyl-carbodiimide multistep reaction, other acids, other olefins (homologation of carboxylic acids); |
-
-
135967-17-8
2-(2-Adamantan-1-yl-1-benzenesulfonyl-1-trimethylsilanyl-ethanesulfinyl)-pyridine

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate; trifluoroacetic acid 2) MeCN, H2O; Multistep reaction; |
-
-
163976-43-0
3-Adamantan-1-yl-2-(pyridine-2-sulfinyl)-propionitrile

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate; trifluoroacetic anhydride 2) EtOH; Multistep reaction; | |
| With potassium carbonate; trifluoroacetic anhydride 2.) acetonitrile; Yield given. Multistep reaction; |
-
-
144499-23-0
Acetic acid 2-adamantan-1-yl-1-cyano-1-(pyridin-2-ylsulfanyl)-ethyl ester

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 25℃; for 12h; Yield given; |

| Conditions | Yield |
|---|---|
| (i) Mg, (ii) /BRN= 1900390/; Multistep reaction; |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In water |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate; trifluoroacetic anhydride 1.) CH2Cl2, 2 h, room temp.; 2.) H2O, acetone, overnight at room temp.; Yield given. Multistep reaction; |

-
A
-
19835-39-3
1-(1-adamantyl)propan-2-one

-
B
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; water |
-
-
19835-38-2
1-adamantylacetyl chloride

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 81 percent / NaOH / toluene / 1 h / 20 °C 2: H2SO4; water View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: DCC / CH2Cl2 2: 33 percent Spectr. / CH2Cl2 / 0 - 5 °C / Irradiation 3: aq.K2CO3 / acetone / 12 h / 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1: DCC / CH2Cl2 2: 79 percent Spectr. / CH2Cl2 / 0 - 5 °C / Irradiation 3: aq.K2CO3 / acetone / 12 h / 25 °C View Scheme | |
| Multi-step reaction with 4 steps 1: DCC / CH2Cl2 2: 87 percent Spectr. / CH2Cl2 / 0 - 5 °C / Irradiation 3: MCPBA 4: 1) (CF3CO)2O, 2) aq.K2CO3 / 2) EtOH View Scheme |
-
-
91233-19-1
1-(adamantane-1-carbonyloxy)pyridine-2(1H)-thione

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 33 percent Spectr. / CH2Cl2 / 0 - 5 °C / Irradiation 2: aq.K2CO3 / acetone / 12 h / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 79 percent Spectr. / CH2Cl2 / 0 - 5 °C / Irradiation 2: aq.K2CO3 / acetone / 12 h / 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 87 percent Spectr. / CH2Cl2 / 0 - 5 °C / Irradiation 2: MCPBA 3: 1) (CF3CO)2O, 2) aq.K2CO3 / 2) EtOH View Scheme | |
| Multi-step reaction with 3 steps 1: 75 percent Spectr. / CH2Cl2 / 0 - 5 °C 2: m-chloroperbenzoic acid 3: 1) CF3CO2H 2) K2CO3 / 2) MeCN, H2O View Scheme | |
| Multi-step reaction with 2 steps 1: 61 percent Spectr. / CH2Cl2 / 0 - 5 °C 2: 1M KOH / methanol View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 1h; Heating; | 100% |
| With sulfuric acid Heating; | 80% |
| With toluene-4-sulfonic acid | |
| With sulfuric acid for 10h; Reflux; |
-
-
81250-34-2
1,3-dipropyl-5,6-diaminouracil

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
136199-23-0
6-amino-1,3-dipropyl-5-<<(1-adamantyl)acetyl>amino>uracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 1,4-dioxane for 2h; Ambient temperature; | 100% |
-
-
16652-71-4
benzyl (2S)-2-pyrrolidinecarboxylate hydrochloride

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
87113-91-5
(S)-1-(2-adamantan-1-yl-acetyl)-pyrrolidine-2-carboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| With N-Ethylmaleimide; benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran | 100% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 18h; | 100% |
| With borane-THF In tetrahydrofuran at 5 - 10℃; | 97% |
| Stage #1: (adamant-1-yl)-acetic acid With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 2h; Inert atmosphere; Stage #2: With Glauber's salt In tetrahydrofuran at 0 - 20℃; for 1h; Inert atmosphere; | 97% |
-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
19835-38-2
1-adamantylacetyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 18h; | 100% |
| With thionyl chloride; N,N-dimethyl-formamide In ethyl acetate at 20℃; for 3h; | 99% |
| With thionyl chloride for 5h; Inert atmosphere; Reflux; | 99% |
-
-
1926-80-3
methyl 6-aminocaproate hydrochloride

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
273201-95-9
Ada-Ahx-OMe

| Conditions | Yield |
|---|---|
| With 6-chloro-3-((dimethylamino)(dimethyliminio)methyl)-1H-benzo[d][1,2,3]triazol-3-ium-1-olatehexafluorophosphate(V); N-ethyl-N,N-diisopropylamine In dichloromethane for 3h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: C32H41N7O2; (adamant-1-yl)-acetic acid With 4-methyl-morpholine; 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dimethylsulfoxide-d6 at 20℃; Stage #2: trifluoroacetic acid In methanol; water | 100% |


| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 20h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 3.5h; | 100% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1,2-dichloro-ethane; N-ethyl-N,N-diisopropylamine In dichloromethane | 99% |
-
-
7517-19-3
methyl (L)-leucinate hydrochloride

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
1040215-19-7
N-(1-adamantylacetyl)-L-leucine methyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 98% |
-
-
933-52-8
2,2,4,4-tetramethyl-1,3-cyclobutanedione

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
1141681-57-3
adamantan-1-ylacetic acid 1-tert-butylcarbamoyl-2,2,4,4-tetramethyl-3-oxocyclobutyl ester

| Conditions | Yield |
|---|---|
| In water at 20℃; for 72h; Passerini reaction; | 98% |

-
-
131326-39-1
2-[2-[[(1,1-dimethylethyl)-dimethylsilyl]oxy]ethoxy]ethanol

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: C27H37N5O3; (adamant-1-yl)-acetic acid With 4-methyl-morpholine; 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dimethylsulfoxide-d6 at 20℃; Stage #2: trifluoroacetic acid In methanol; water | 98% |

-
-
40615-39-2
5'-O-(4-4'-dimethoxytrityl)thymidine

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Molecular sieve; | 97% |
-
-
139115-91-6
2-(2-tert-butyloxycarbonylaminoethoxy)ethanol

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-tert-butyloxycarbonylaminoethoxy)ethanol; (adamant-1-yl)-acetic acid With dmap In dichloromethane at 20℃; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; | 97% |
| With dmap In dichloromethane at 0 - 20℃; Inert atmosphere; | 97% |
| In dichloromethane at 0 - 20℃; | 97% |
| With benzotriazol-1-ol; 1,2-dichloro-ethane In dichloromethane |
-
-
97315-19-0
3-<(tert-butyldimethylsilyl)oxy>-4-methoxybenzyl alkohol

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 24h; | 96% |
-
-
75-35-4
1,1-Dichloroethylene

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
17768-28-4
1,3-adamantane diacetic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid for 5h; Ambient temperature; | 95% |
-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
17768-34-2
(3-bromo-1-adamantyl)acetic acid

| Conditions | Yield |
|---|---|
| With water; bromine for 1.5h; Heating; | 94% |
| With bromine | |
| With sulfuric acid; hydrogen bromide | |
| With bromine In tetrachloromethane | |
| With t-butyl bromide; sulfuric acid In tetrachloromethane at 10 - 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With water; bromine 1.) reflux, 15 min, 2.) room temp., overnight; | 94% |
-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: (adamant-1-yl)-acetic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: With sodium hydrogen sulfide In N,N-dimethyl-formamide at 20℃; | 94% |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 75℃; for 12h; Inert atmosphere; | 94% |

-
-
4942-47-6
(adamant-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: (adamant-1-yl)-acetic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 1h; Stage #2: 4-(4-(aminomethyl)phenoxy)phthalonitrile In dichloromethane for 10h; | 93.8% |
-
-
64-17-5
ethanol

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
15782-66-8
ethyl 4-(adamant-1-yl)-phenyl acetate

| Conditions | Yield |
|---|---|
| With sulfuric acid for 5h; Reflux; | 93% |
-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
1673-47-8
3-chlorobenzhydrazide

-
-
1417863-11-6
2-(1-adamantylmethyl)-5-(3-chlorophenyl)-1,3,4-oxadiazole

| Conditions | Yield |
|---|---|
| Stage #1: (adamant-1-yl)-acetic acid; 3-chlorobenzhydrazide With trichlorophosphate for 4h; Reflux; Stage #2: With potassium carbonate; potassium hydroxide In water pH=8; Cooling with ice; | 93% |
-
-
109-73-9
N-butylamine

-
-
4942-47-6
(adamant-1-yl)-acetic acid

-
-
122020-51-3
2-(adamantan-1-yl)-N-butylacetamide

| Conditions | Yield |
|---|---|
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; | 92% |
| With boron trifluoride diethyl etherate; triethylamine In toluene Heating; | 77% |
1-Adamantaneacetic acid Chemical Properties
Molecule structure of 1-Adamantaneacetic acid (CAS NO.4942-47-6):
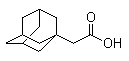
IUPAC Name: 2-(1-Adamantyl)acetic acid
Molecular Weight: 194.27012 g/mol
Molecular Formula: C12H18O2
Density: 1.167 g/cm3
Melting Point: 134-137 °C(lit.)
Boiling Point: 319.2 °C at 760 mmHg
Flash Point: 163 °C
Index of Refraction: 1.55
Molar Refractivity: 53.01 cm3
Molar Volume: 166.4 cm3
Surface Tension: 52 dyne/cm
Enthalpy of Vaporization: 61.65 kJ/mol
Vapour Pressure: 7.23E-05 mmHg at 25 °C
XLogP3-AA: 3.2
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 2
Exact Mass: 194.13068
MonoIsotopic Mass: 194.13068
Topological Polar Surface Area: 37.3
Heavy Atom Count: 14
Canonical SMILES: C1C2CC3CC1CC(C2)(C3)CC(=O)O
InChI: InChI=1S/C12H18O2/c13-11(14)7-12-4-8-1-9(5-12)3-10(2-8)6-12/h8-10H,1-7H2,(H,13,14)
InChIKey: AOTQGWFNFTVXNQ-UHFFFAOYSA-N
EINECS: 225-585-1
Product Categories: cyclic compounds; Adamantane derivatives; Adamantanes
1-Adamantaneacetic acid Safety Profile
Hazard Codes:  Xi,
Xi,  Xn,
Xn,  F
F
Risk Statements: 36/37/38-15-10
R36/37/38:Irritating to eyes, respiratory system and skin.
R15:Contact with water liberates extremely flammable gases.
R10:Flammable.
Safety Statements: 22-24/25-43-7/8
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
S43:In case of fire use ... (there follows the type of fire-fighting equipment to be used.)
S7:Keep container tightly closed.
S8:Keep container dry.
WGK Germany: 3
HazardClass: IRRITANT
1-Adamantaneacetic acid Specification
1-Adamantaneacetic acid (CAS NO.4942-47-6) is also named as Adamantan-1-ylacetic acid ; tricyclo[3.3.1.1~3,7~]decane-1-acetic acid ; 2-(1-adamantyl)acetic acid ; Tricyclo(3.3.1.13,7)dec-1-ylacetic acid ; Tricyclo[3.3.1.1(3,7)-]decane-1-acetic acid . 1-Adamantaneacetic acid (CAS NO.4942-47-6) is white to off-white crystalline powder or crystals.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View