-
Name
1-Benzyl-2-pyrrolidinone
- EINECS 226-131-5
- CAS No. 5291-77-0
- Article Data84
- CAS DataBase
- Density 1.134 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 154 - 156oC
- Formula C11H13NO
- Boiling Point 343.041 °C at 760 mmHg
- Molecular Weight 175.23
- Flash Point 141.343 °C
- Transport Information
- Appearance clear colourless to yellow liquid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Pyrrolidinone,1-(phenylmethyl)-;2-Pyrrolidinone,1-benzyl- (6CI,7CI,8CI);1-Benzyl-2-oxopyrrolidine;1-Benzylazacyclopentan-2-one;N-Benzyl-2-pyrrolidone;N-Benzylbutyrolactam;N-Benzylpyrrolidin-2-one;N-Benzylpyrrolidone;NSC 30184;
- PSA 20.31000
- LogP 1.74690
Synthetic route
-
-
534616-88-1
1-benzylazetidine-2-carbaldehyde

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With 2-pentafluorophenyl-6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4]triazol-2-ium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium Tetrafluoroborate In 1,4-dioxane at 220℃; for 0.583333h; Microwave irradiation; | 99% |
| for 24h; Heating; | 79% |
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; sodium acetate In toluene at 110℃; for 36h; Reagent/catalyst; Inert atmosphere; Glovebox; Molecular sieve; Schlenk technique; | 65% |
-
-
64017-86-3
N-benzyl-4-(benzylamino)butanamide

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; sodium acetate; benzylamine In toluene for 48h; Inert atmosphere; Glovebox; Molecular sieve; Schlenk technique; Reflux; | 97% |
-
-
22813-61-2
N-benzyl-4-chlorobutanamide

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; for 2h; | 96% |
| With sodium hydride In hexane; N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; | 96% |
| With potassium hydroxide |
-
-
1307661-09-1
C18H18ClN

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With tetrakis(trifluoroacetato)rhodium(II); water In toluene for 10h; Reflux; Inert atmosphere; chemoselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen; sodium sulfate; palladium on activated charcoal In ethyl acetate at 100℃; under 30002.4 Torr; for 4h; | 93% |
| With hydrogen; sodium sulfate; palladium on activated charcoal In ethyl acetate at 100℃; under 30002.4 Torr; for 4h; | 93% |

| Conditions | Yield |
|---|---|
| With N-benzyl-trimethylammonium hydroxide at 20℃; Neat (no solvent); chemoselective reaction; | 92% |
| Stage #1: 2-pyrrolidinon With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran; mineral oil at 0 - 23℃; for 15h; Inert atmosphere; | 83% |
| Stage #1: 2-pyrrolidinon With sodium hydride In tetrahydrofuran; paraffin oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran; paraffin oil at 0 - 20℃; Inert atmosphere; | 82% |

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In diethyl ether at 0℃; for 0.25h; Inert atmosphere; | 91.5% |

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene at 10℃; for 2h; Irradiation; | 91% |
-
-
35665-25-9
cyclopropanecarboxylic acid benzylamide

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In acetonitrile at 60℃; for 10h; | 91% |

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile for 48h; Heating; | 90% |
| potassium fluoride on basic alumina In 1,2-dimethoxyethane for 48h; Ambient temperature; | 81% |
| With potassium carbonate; tetrabutylammomium bromide In toluene at 80℃; for 24h; Product distribution; Mechanism; Rate constant; var. time, catalyst; effect of mixing, carbonate and water; | 76% |
-
-
26735-10-4
4-(benzylamino)butanoic acid

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With trimethylsilylethoxyacetylene; toluene-4-sulfonic acid; mercury(II) oxide In water; 1,2-dichloro-ethane; acetonitrile at 60℃; for 42h; | 87% |
-
-
80251-79-2
(5R)-<5-2H1>-2-pyrrolidinone

-
-
100-39-0
benzyl bromide

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
102608-50-4
(5R)-<5-(2)H1>-1-benzylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether for 1h; Heating; | A n/a B 85% |

-
-
19340-88-6
N-benzyl-4-hydroxybutanamide

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; sodium acetate; benzylamine In toluene for 48h; Inert atmosphere; Glovebox; Molecular sieve; Schlenk technique; Reflux; | 79% |
| sulfuric acid for 2h; Heating; |
-
-
74798-56-4
1-benzyl-2-methylpiperidin-3-one

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
138052-86-5
1-benzyl-2-acetylpyrrolidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 24h; Heating; | A n/a B 78% |
-
-
96-48-0
4-butanolide

-
-
100-46-9
benzylamine

-
A
-
19340-88-6
N-benzyl-4-hydroxybutanamide

-
B
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| at 150℃; for 0.25h; Microwave irradiation; | A 78% B 8% |

-
-
41194-02-9
1-benzyl-5-hydroxypyrrolidin-2-one

-
-
140-88-5
ethyl acrylate

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
1393803-16-1
1-benzyl-5-[2-(ethyloxycarbonyl)ethyl]pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With samarium diiodide; boron trifluoride diethyl etherate; tert-butyl alcohol In tetrahydrofuran at -40℃; for 0.166667h; | A 10% B 78% |


| Conditions | Yield |
|---|---|
| With iron(III) chloride In acetonitrile at 80℃; Sealed tube; | 78% |
-
-
41194-02-9
1-benzyl-5-hydroxypyrrolidin-2-one

-
-
1663-39-4
tert-Butyl acrylate

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
1393803-37-6
1-benzyl-5-[2-(tert-butyloxycarbonyl)ethyl]pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With samarium diiodide; boron trifluoride diethyl etherate; tert-butyl alcohol In tetrahydrofuran at -40℃; for 0.166667h; | A 8% B 76% |


| Conditions | Yield |
|---|---|
| Stage #1: carbon monoxide; 1,3-dibenzyl-1-cyclopropylurea With bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; p-N,N-dimethylaminobenzoic acid; triphenylphosphine In 1,2-dichloro-benzene at 130℃; for 72h; Stage #2: With hydrogen; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,2-dichloro-benzene at 140℃; for 36h; | 75% |
-
-
41194-02-9
1-benzyl-5-hydroxypyrrolidin-2-one

-
-
97-63-2
methyl methacrylate

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With samarium diiodide; boron trifluoride diethyl etherate; tert-butyl alcohol In tetrahydrofuran at -40℃; for 0.166667h; | A 17% B 73% |


| Conditions | Yield |
|---|---|
| With N-methyl-2-pyrrolidone hydrochloride; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; palladium(II) iodide at 120℃; under 22801.5 Torr; for 24h; Autoclave; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: N-benzylcyclopropanamine With isothiocyanatocyclohexane In 1,2-dichloro-benzene at 80℃; for 3h; Inert atmosphere; Stage #2: carbon monoxide With bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; 3-phenoxypropanoic acid; triphenylphosphine In 1,2-dichloro-benzene at 130℃; for 20h; | 70% |
-
-
201230-82-2
carbon monoxide

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; 3-phenoxypropanoic acid; triphenylphosphine In 1,2-dichloro-benzene at 130℃; for 72h; | 68% |
-
-
14468-90-7
N-trimethylsilyl-pyrrolidin-2-one

-
-
100-39-0
benzyl bromide

-
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| trimethylsilyl trifluoromethanesulfonate at 150 - 160℃; for 0.5h; | 66% |

-
-
100-52-7
benzaldehyde

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
94914-32-6
methyl 4-(dibenzylamino)butanoate

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; triethylamine In dichloromethane | A 65% B 20% |

-
-
41194-02-9
1-benzyl-5-hydroxypyrrolidin-2-one

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With samarium diiodide; boron trifluoride diethyl etherate; tert-butyl alcohol In tetrahydrofuran at -40℃; for 0.166667h; Overall yield = 49 %; | A 65% B n/a C n/a |

-
-
41194-02-9
1-benzyl-5-hydroxypyrrolidin-2-one

-
-
107-13-1
acrylonitrile

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
1393803-38-7
1-benzyl-5-(2-cyanoethyl)pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With samarium diiodide; boron trifluoride diethyl etherate; tert-butyl alcohol In tetrahydrofuran at -40℃; for 0.166667h; | A 15% B 54% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; carbonylhydridetris(triphenylphosphine)rhodium(I) In dichloromethane; isopropyl alcohol at 100℃; under 26220 Torr; for 24h; | 51% |
-
-
79583-98-5
5-azidovaleric acid

-
-
71-43-2
benzene

-
A
-
5291-77-0
1-benzyl-2-pyrrolidone

-
B
-
3389-54-6
n-benzoylpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: 5-azidovaleric acid With oxalyl dichloride In dichloromethane at 30℃; for 1.5h; Schmidt Reaction; Stage #2: benzene With trifluorormethanesulfonic acid In dichloromethane at 0 - 60℃; for 46h; Schmidt Reaction; | A 35% B 51% |

-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
29897-82-3
N-benzylpyrrolidine

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer In tetrahydrofuran at 65℃; for 1h; | 96% |
| With sodium tetrahydroborate; titanium tetrachloride In 1,2-dimethoxyethane for 14h; Ambient temperature; | 93% |
| With Dimethylphenylsilane In ethyl acetate at 20 - 80℃; for 24h; Green chemistry; | 93% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
201218-09-9
cyclopropyl acetylenyllithium

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With 2,6-di-tert-butyl-4-methylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at -78℃; for 0.5h; Inert atmosphere; Stage #2: cyclopropyl acetylenyllithium In tetrahydrofuran; dichloromethane at -78 - 20℃; for 1h; Inert atmosphere; | 96% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
17642-88-5
1-benzyl-2-pyrrolidinethione

| Conditions | Yield |
|---|---|
| With Lawessons reagent In dichloromethane at 20℃; | 95% |
| With Lawessons reagent In dichloromethane at 20℃; Inert atmosphere; | 90% |
| With Lawessons reagent In benzene for 4h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With 2,6-di-tert-butyl-4-methylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at -78℃; for 0.5h; Inert atmosphere; Stage #2: lithium phenylacetylide In tetrahydrofuran; dichloromethane at -78 - 20℃; for 1h; Reagent/catalyst; Inert atmosphere; | 95% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
60765-09-5
1-lithio-1-nonyne

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With 2,6-di-tert-butyl-4-methylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at -78℃; for 0.5h; Inert atmosphere; Stage #2: 1-lithio-1-nonyne In tetrahydrofuran; dichloromethane at -78 - 20℃; for 1h; Inert atmosphere; | 95% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
762-04-9
phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone; trifluoromethylsulfonic anhydride With 2,6-di-tert-butyl-4-methylpyridine In dichloromethane at -78 - 0℃; Stage #2: phosphonic acid diethyl ester In dichloromethane at 0℃; for 5h; | 93% |


| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In toluene at 70℃; for 16h; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With lithium diisopropyl amide In tetrahydrofuran; hexane at -78 - -60℃; for 0.666667h; Inert atmosphere; Stage #2: methyl 3-methylbutanoate In tetrahydrofuran; hexane at -78 - 20℃; for 12h; Inert atmosphere; | 92% |
| Stage #1: 1-benzyl-2-pyrrolidone With n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; Stage #2: methyl 3-methylbutanoate In tetrahydrofuran at -78 - 30℃; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With tin(IV) chloride; trichlorophosphate In tetrahydrofuran; chloroform at 20℃; for 1.5h; Stage #2: anthranilic acid nitrile In tetrahydrofuran; chloroform at 50℃; for 5h; | 91% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
75-16-1
methylmagnesium bromide

-
-
220024-87-3
1-benzyl-2,2-dimethylpyrrolidine

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride In tetrahydrofuran; diethyl ether 1.) -10 deg C, 0.5 h, 2.) 0 deg C to r.t., 4 h; | 90% |

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In toluene at 70℃; for 16h; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With lithium diisopropyl amide In tetrahydrofuran; hexane at -78 - -60℃; for 0.666667h; Inert atmosphere; Stage #2: propanoic acid methyl ester In tetrahydrofuran; hexane at -78 - 20℃; for 12h; Inert atmosphere; | 88% |
| Stage #1: 1-benzyl-2-pyrrolidone With n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; Stage #2: propanoic acid methyl ester In tetrahydrofuran at -78 - 30℃; | 61% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
848414-09-5
5-methanesulfonyl-furan-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.783333h; Stage #2: 5-methanesulfonyl-furan-2-carboxylic acid ethyl ester In tetrahydrofuran at -78 - 20℃; for 1.83333h; | 87% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
848414-09-5
5-methanesulfonyl-furan-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.783333h; Stage #2: 5-methanesulfonyl-furan-2-carboxylic acid ethyl ester In tetrahydrofuran at -78 - 20℃; for 1.83333h; | 87% |
-
-
5291-77-0
1-benzyl-2-pyrrolidone

-
-
925-90-6
ethylmagnesium bromide

-
-
1228468-95-8
1-benzyl-2,2-diethylpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-2-pyrrolidone With 2,6-di-tert-butyl-4-methylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at -78℃; for 0.75h; Stage #2: ethylmagnesium bromide In diethyl ether; dichloromethane at -78 - 20℃; | 87% |
| Stage #1: 1-benzyl-2-pyrrolidone With 2,6-di-tert-butyl-4-methylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at -78℃; for 0.75h; Inert atmosphere; Stage #2: ethylmagnesium bromide In dichloromethane at -78 - 20℃; for 3h; Reagent/catalyst; Inert atmosphere; | 87% |
1-Benzyl-2-pyrrolidinone Specification
The 1-Benzyl-2-pyrrolidinone, with the CAS registry number 5291-77-0, is also known as N-Benzyl-2-pyrrolidone. It belongs to the product categories of Heterocyclic Building Blocks; Pyrrolidines. Its EINECS number is 226-131-5. This chemical's molecular formula is C11H13NO and molecular weight is 175.23. What's more, its systematic name is Methyl 2,6-difluorobenzoate. Its classification codes are: (1)Antineoplastic agents; (2)Drug / Therapeutic Agent; (3)Immunologic Factors; (4)Interferon inducers; (5)Mutation data; (6)Reproductive Effect. This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from light.
Physical properties of 1-Benzyl-2-pyrrolidinone are: (1)ACD/LogP: 0.745; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.75; (4)ACD/LogD (pH 7.4): 0.75; (5)ACD/BCF (pH 5.5): 2.17; (6)ACD/BCF (pH 7.4): 2.17; (7)ACD/KOC (pH 5.5): 60.58; (8)ACD/KOC (pH 7.4): 60.58; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 20.31 Å2; (13)Index of Refraction: 1.578; (14)Molar Refractivity: 51.31 cm3; (15)Molar Volume: 154.578 cm3; (16)Polarizability: 20.341×10-24cm3; (17)Surface Tension: 47.6 dyne/cm; (18)Density: 1.134 g/cm3; (19)Flash Point: 141.343 °C; (20)Enthalpy of Vaporization: 58.686 kJ/mol; (21)Boiling Point: 343.041 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
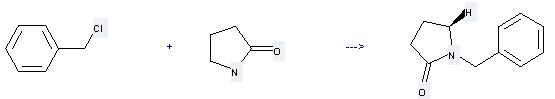
Preparation: this chemical can be prepared by pyrrolidin-2-one and chloromethyl-benzene at the ambient temperature. This reaction will need solvent 1,2-dimethoxy-ethane with the reaction time of 48 hours. This reaction will also need catalyst KF-alumina. The yield is about 81%.
Uses of 1-Benzyl-2-pyrrolidinone: it can be used to produce 1-benzyl-pyrrolidine at the ambient temperature. It will need reagents TiCl4, NaBH4 and solvent 1,2-dimethoxy-ethane with the reaction time of 14 hours. The yield is about 93%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C2N(Cc1ccccc1)CCC2
(2)Std. InChI: InChI=1S/C11H13NO/c13-11-7-4-8-12(11)9-10-5-2-1-3-6-10/h1-3,5-6H,4,7-9H2
(3)Std. InChIKey: LVUQCTGSDJLWCE-UHFFFAOYSA-N
Related Products
- 1-Benzyl-2-pyrrolidinone
- 52918-59-9
- 52918-63-5
- 52918-86-2
- 529-19-1
- 529-20-4
- 5292-13-7
- 5292-21-7
- 529-23-7
- 5292-42-2
- 5292-43-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View