-
Name
1-Bromooctane
- EINECS 203-912-9
- CAS No. 111-83-1
- Article Data154
- CAS DataBase
- Density 1.111 g/cm3
- Solubility insoluble
- Melting Point -55 °C
- Formula C8H17Br
- Boiling Point 200.9 °C at 760 mmHg
- Molecular Weight 193.127
- Flash Point 78.3 °C
- Transport Information
- Appearance clear colourless to yellow-brown liquid
- Safety 23-24/25
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols 36/37/38:;
- Synonyms 1-Bromo Octane;1-Octylbromide;Octyl bromide;Octane, 1-bromo-;N-bromooctane;
- PSA 0.00000
- LogP 3.74180
Synthetic route

| Conditions | Yield |
|---|---|
| With dibromobis(cyclopentadienyl)titanium(IV); triisobutylaluminum; 1,2-dibromomethane In hexane at 20℃; for 24h; Reagent/catalyst; | 99% |
| With hydrogen bromide; cetyltributylphosphonium bromide at 130℃; for 8.5h; | 86% |
| With magnesium bromide In diethyl ether at 25℃; for 10h; | 41 % Chromat. |
| With aluminum tri-bromide In chlorobenzene at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; polystyrene-supported triphenylphosphine In chloroform at 20℃; for 0.333333h; | 98% |
| With sulfuric acid; hydrogen bromide In water at 60℃; Temperature; Flow reactor; Green chemistry; | 98% |
| With 1-(2-OPPh2-propyl)-3-methylimidazolium hexafluorophosphate; bromine at 80℃; for 2h; | 96% |
-
-
103514-65-4
C14H35N3PS(1+)

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With bromide In N,N-dimethyl-formamide | 96% |
-
-
16156-52-8
n-octyl methanesulfonate

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With potassium bromide; O3Si(CH2)3P(C4H9)3Br; silica gel In carbon dioxide; water at 70℃; under 97509.8 Torr; for 5h; | 95% |
| With potassium bromide; tributylphosphonium salts bonded to .. In water; toluene at 60℃; Rate constant; variation of catalists; | |
| With 2-hexadecyl-1,3-bis-(1,4,7,10-tetraoxa-13-azacyclopentadecan-13-yl)propane; sodium bromide In toluene at 50℃; Rate constant; also with KBr; further phase-transfer catalysts; |

| Conditions | Yield |
|---|---|
| With hydrogen bromide In hexane at 24.9℃; for 48h; Mechanism; Product distribution; other reagent concentrations and other reaction temperatures; | A 95% B 5% |
| With borane; bromine; sodium methylate 1.) THF, 0 deg C, 2.) 0 deg C, 3.) MeOH, 0 deg C; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide at 50℃; for 5h; Inert atmosphere; Green chemistry; | 93% |
| With lithium bromide |
-
-
88738-43-6
methoxymethyl octyl ether

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; tetrabutylammomium bromide at 130 - 142℃; for 0.0333333h; Microwave irradiation; Ionic liquid; chemoselective reaction; | 91% |
| With tetrabutylammomium bromide; 1-(n-butyl)-3-methylimidazolium tetrachloroindate at 135 - 140℃; for 0.0666667h; Microwave irradiation; Neat (no solvent); chemoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; phosphorus tribromide at 125℃; for 0.666667h; | 90% |

| Conditions | Yield |
|---|---|
| With calcium bromide; tetrahexylammonium bromide at 110℃; for 24h; | 89% |
| With pyridine; tributyltin bromide at 100℃; Thermodynamic data; Equilibrium constant; Δ G; | |
| With lithium bromide; tetrahexylammonium bromide In water at 98℃; for 72h; various metal halide salts; bromine-chloride exchange; |
-
-
796965-95-2
ethoxymethyl octyl ether

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; tetrabutylammomium bromide at 130 - 142℃; for 0.0333333h; Microwave irradiation; Ionic liquid; chemoselective reaction; | 89% |
| With tetrabutylammomium bromide; 1-(n-butyl)-3-methylimidazolium tetrachloroindate at 135 - 140℃; for 0.0666667h; Microwave irradiation; Neat (no solvent); chemoselective reaction; | 83% |

| Conditions | Yield |
|---|---|
| With iodobenzene dibromide In dichloromethane for 1h; Ambient temperature; | 85% |
| With pyridine; tributyltin bromide at 100℃; Thermodynamic data; Equilibrium constant; Δ G; | 36 % Spectr. |
| With n-hexylzirconocene chloride; [nickel(II) (4,4'-di-tert-butyl-2,2'-bipyridine)(bromide)2]; tetrabutylammonium p-toluenesulfonate In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; Irradiation; Sealed tube; |
-
-
14246-16-3
trimethyl(oct-1-yloxy)silane

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With trimethylsilyl bromide; iodine(I) bromide for 6h; Heating; | 85% |
-
-
54384-75-7
1-methoxy-4-octyloxymethyl benzene

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 0 - 30℃; for 2.5h; | 85% |
-
-
52770-75-9
1,3-Diisopropyl-2-octyl-isourea

-
A
-
4128-37-4
1,3-diisopropylurea

-
B
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; tetrabutylammomium bromide In chloroform at 65℃; for 7.5h; | A n/a B 83% |

| Conditions | Yield |
|---|---|
| Stage #1: oct-1-ene With dibenzoyl peroxide at 30℃; for 0.5h; Stage #2: With hydrogen bromide at 20℃; for 0.5h; Temperature; | 82.2% |
| With hydrogenchloride; borane; chloroamine-T; sodium bromide 1.) 0 deg C, 1 h, THF, 2.) 0 deg C, 15 min, H2O, THF; Yield given. Multistep reaction; | |
| With hydrogen bromide In hexane at -78.1℃; for 48h; Yield given; | |
| Multi-step reaction with 2 steps 1: bis(1,5-cyclooctadiene)diiridium(I) dichloride / dichloromethane / 16 h / 0 - 20 °C / Inert atmosphere; Glovebox 2: benzenesulfonyl bromide; trimethylsilyl trifluoromethanesulfonate; 2-methoxybenzo[d][1,3,2]dioxaborole / dichloromethane; benzene; methanol / 16 h / 70 °C / Inert atmosphere View Scheme |
-
-
76207-90-4
5,6,8,9-tetrahydro-14-octyl-7-phenyldibenzoacridinium bromide

-
A
-
57366-68-4
5,6,8,9-tetrahydro-7-phenyldibenzolacridine

-
B
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With acetonitrile 1.) reflux, 4 h, 2.) 0 deg C, 6 h; | A n/a B 80% |

| Conditions | Yield |
|---|---|
| With phosphorus tribromide In diethyl ether at 0 - 25℃; for 6h; | A 12.2% B 77.3% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; triphenylphosphine In dichloromethane at 20℃; for 12h; | 75% |
-
-
66217-56-9
4,4,5,5-tetramethyl-2-octyl-[1,3,2]dioxaborolane

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 2-methoxybenzo[d][1,3,2]dioxaborole; benzenesulfonyl bromide In methanol; dichloromethane; benzene at 70℃; for 16h; Inert atmosphere; | 66% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; phosphorus tribromide at 50℃; for 0.666667h; | A 15% B 50% |
-
-
7333-16-6
4-hydroxybutyl octyl sulfoxide

-
A
-
111-83-1
1-bromo-octane

-
B
-
33036-62-3
4-Bromo-1-butanol

-
D
-
80623-45-6
4-bromobutyl octyl sulfone

| Conditions | Yield |
|---|---|
| With bromine In acetonitrile at -25℃; for 2h; Further byproducts given; | A n/a B n/a C 24% D 46% |

-
-
68975-45-1
1-(methylethoxy)octane

-
A
-
111-83-1
1-bromo-octane

-
B
-
35103-75-4
mono-1-methylheptyl phosphonate

| Conditions | Yield |
|---|---|
| With hydrogen bromide; phosphorus tribromide at 80℃; for 0.666667h; | A 45% B 9% |

| Conditions | Yield |
|---|---|
| With bromine In acetonitrile at -25℃; for 2h; Product distribution; | A n/a B n/a C n/a D n/a E n/a F 24% |

-
-
67007-46-9
n-octylsilatrane

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dichloromethane for 78h; Ambient temperature; | 15% |
-
-
111-66-0
oct-1-ene

-
-
75-26-3
isopropyl bromide

-
A
-
7642-04-8
cis-2-octene

-
B
-
13389-42-9
trans-2-Octene

-
C
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| mit UV-Licht.Irradiation; |

-
-
16156-52-8
n-octyl methanesulfonate

-
-
14866-33-2
tetraoctyl ammonium bromide

-
A
-
111-83-1
1-bromo-octane

-
B
-
70098-05-4
tetraoctylammonium methanesulfonate

| Conditions | Yield |
|---|---|
| In chlorobenzene at 60℃; Rate constant; | |
| In water; chlorobenzene at 60℃; Kinetics; |

-
-
16156-52-8
n-octyl methanesulfonate

-
-
92958-33-3
3-Tetradecyl-4,10,15-trioxa-1,7-diaza-bicyclo[5.5.5]heptadecane; hydrobromide

-
A
-
111-83-1
1-bromo-octane

-
B
-
80322-81-2
3-Tetradecyl-4,10,15-trioxa-1,7-diaza-bicyclo[5.5.5]heptadecane; compound with methanesulfonic acid

| Conditions | Yield |
|---|---|
| In chlorobenzene at 60℃; Rate constant; |

-
-
16156-52-8
n-octyl methanesulfonate

-
A
-
111-83-1
1-bromo-octane

-
B
-
629-82-3
di-n-octyl ether

-
C
-
2386-56-3
potassium mesylate

| Conditions | Yield |
|---|---|
| With potassium bromide; perhydrodibenzo-18-crown-6 In potassium hydroxide; chlorobenzene at 60℃; for 1h; | A 94.4 % Chromat. B 3 % Chromat. C n/a |


| Conditions | Yield |
|---|---|
| With potassium bromide In water; benzene at 45℃; gaschromatographic determination of formation of octyl bromide in dependence on time, addition of various quaternary onium salts; also with solvent CHCl3/H2O; two phase system and homogeneous system; | |
| With potassium bromide; (C16H33)Bu3P(1+)*Br(1-) In water; chlorobenzene at 60℃; Rate constant; other quaternary ammonium or phosphonium; | |
| With potassium bromide; (C8H17)4N(1+)*Br(1-) In water; chlorobenzene at 35℃; Rate constant; other solvents; |
-
-
111-87-5
octanol

-
-
81106-32-3
dimethylamino-2,N,N' diphenyl-1,3 diazaphospholane-1,3,2

-
-
111-83-1
1-bromo-octane

| Conditions | Yield |
|---|---|
| With bromine 1.) toluene, 85 deg C, 18 h 12.) CH2Cl2, 0 deg C; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: thiophene With n-butyllithium In tetrahydrofuran; hexane at -80℃; for 0.25h; Inert atmosphere; Stage #2: 1-bromo-octane In tetrahydrofuran; hexane at -80 - 20℃; Stage #3: With water In tetrahydrofuran; hexane | 100% |
| Stage #1: thiophene With n-butyllithium In tetrahydrofuran; hexane at -78℃; Stage #2: 1-bromo-octane In tetrahydrofuran; hexane at -78 - 20℃; | 95% |
| Stage #1: thiophene With n-butyllithium In tetrahydrofuran; hexane at 0 - 20℃; Inert atmosphere; Stage #2: 1-bromo-octane In tetrahydrofuran; hexane for 4h; Inert atmosphere; Reflux; | 84.6% |

| Conditions | Yield |
|---|---|
| C(CH2S(=O)C6H13)4 at 100℃; for 0.5h; | 100% |
| With silica gel at 80℃; for 8h; | 97% |
| With 2-methylsulfinylpyridine In xylene at 100℃; for 40h; | 96% |

| Conditions | Yield |
|---|---|
| poly<1-hydroxyethylene-co-1-(2-ethylsulf In 1,4-dioxane at 75℃; for 23h; Product distribution; Rate constant; other catalysts; | 100% |
| Sucrose-ethyleneoxide adducts In toluene at 110℃; for 8h; Product distribution; further catalysts: PEG, DB18K6; further objects of study: phase-transfer catalysis; | 100% |
| dodecyldimethylsulfonium methyl sulfate In water at 40℃; for 6h; | 25% |

| Conditions | Yield |
|---|---|
| Sucrose-ethyleneoxide adducts In acetonitrile at 83℃; for 24h; Product distribution; further catalysts: PEG, DB18K6; further objects of study: phase-transfer catalysis; further solvents: toluene/H2O; | 100% |
| tetraoctyl ammonium bromide In water; toluene at 90℃; for 24h; Product distribution; other phase-transfer catalysts, time; | 99% |
| tetraoctyl ammonium bromide In water; toluene at 90℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium iodide; Sucrose-ethyleneoxide adducts In toluene at 110℃; for 24h; Product distribution; further catalysts: PEG, DB18K6; further objects of study: phase-transfer catalysis; further solvents: toluene/H2O; | 100% |
| With potassium iodide; polymeric crown ethers In water; toluene at 79.9℃; for 5h; Product distribution; catalysts with other crown-ether groups; or NaI, other reaction times; | 98% |
| With potassium iodide; tetraoctyl ammonium bromide In water; toluene at 90℃; for 24h; | 97% |

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide at 20℃; for 12h; | 100% |
| With sodium azide In water; acetone Reflux; | 100% |
| With sodium azide In methanol at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In chlorobenzene for 48h; Heating; | 100% |
| In toluene Reflux; | 100% |
| In o-xylene Heating; | 98.7% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide for 0.25h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate Williamson's etherification; | 100% |
| With potassium carbonate In acetone for 43h; Heating; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 48h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide for 0.5h; Heating; | 100% |
-
-
111-83-1
1-bromo-octane

-
-
55716-09-1
S,S'-di-n-butyl dithiocarbonate

-
-
16900-07-5
1-butyl 1-octyl sulphide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide for 1.5h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide for 3h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Sucrose-ethyleneoxide adducts In acetonitrile at 83℃; for 24h; Product distribution; further catalysts: PEG, DB18K6; further objects of study: phase-transfer catalysis; further solvents: toluene/H2O; | 100% |
| With aluminum oxide at 196℃; for 0.0333333h; Irradiation; other long chain halides; | 99% |
| With aluminum oxide at 196℃; for 0.0333333h; Irradiation; | 99% |

| Conditions | Yield |
|---|---|
| Aliquat 336 at 70℃; for 1.5h; | 100% |
-
-
111-83-1
1-bromo-octane

-
-
119289-79-1
3-(4-hydroxy-2-nitrophenyl)pyridine

-
-
158461-43-9
3-(2-nitro-4-n-octyloxyphenyl)pyridine

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 70℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Heating; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 160℃; for 0.5h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride for 0.5h; Irradiation; | 100% |
-
-
111-83-1
1-bromo-octane

-
-
94-09-7
p-aminoethylbenzoate

-
-
55791-74-7
4-octylamino-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 125℃; for 10h; Addition; | 100% |
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 125℃; for 24h; | 65% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium fluoride; potassium hydroxide In acetonitrile at 20℃; for 2h; Product distribution; effect of various catalyst and solvents; | 100% |
| With potassium hydroxide; Aliquat 336 at 20℃; for 2h; | 100% |
-
-
111-83-1
1-bromo-octane

-
-
1707-03-5
diphenyl-phosphinic acid

-
-
3389-73-9
octyl phenyl(phenyl)phosphinate

| Conditions | Yield |
|---|---|
| With potassium carbonate at 140℃; for 10h; | 100% |
| With caesium carbonate In acetonitrile at 100℃; for 12h; Inert atmosphere; | 93% |
-
-
111-83-1
1-bromo-octane

-
-
156747-01-2
5,17,29-tri-tert-butyl-37,39,41-trihydroxy-38,40,42-trimethoxycalix[6]arene

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 90℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| Darkness; | 100% |
| Darkness; | 100% |
| In acetonitrile at 70℃; | 97% |
-
-
111-83-1
1-bromo-octane

-
-
2150-44-9
1-carbomethoxy-3,5-dihydroxybenzene

-
-
87963-84-6
methyl 3,5-bis(octyloxy)benzoate

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In acetone for 60h; Etherification; Heating; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 15h; Inert atmosphere; | 98% |
| With adogen 464; potassium carbonate In acetone Heating; | 96% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol; N,N-dimethyl-formamide at 100℃; for 72h; | 100% |
| With potassium carbonate In acetonitrile at 85℃; Sealed tube; Inert atmosphere; | 100% |
| With sodium methylate In methanol for 48h; Alkylation; Heating; | 79% |
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 24h; | 71% |
| With sodium hydroxide In acetonitrile at 20℃; for 24h; Reagent/catalyst; | 76 g |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 60℃; for 24h; Inert atmosphere; | 100% |
| In neat (no solvent) for 5h; Sonication; Reflux; | 98% |
| at 80℃; for 72h; | 97% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 15h; Heating; | 100% |
| In acetone for 9h; Heating; | 50% |
| In ethyl acetate at 70℃; for 48h; |
-
-
111-83-1
1-bromo-octane

-
-
6089-04-9
3-(tetrahydropyran-2'-yloxy)propyne

-
-
88517-99-1
2-undecynyltetrahydro-2H-pyran-2-yl ether

| Conditions | Yield |
|---|---|
| Stage #1: 3-(tetrahydropyran-2'-yloxy)propyne With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide at -78℃; Stage #2: 1-bromo-octane In tetrahydrofuran | 100% |
| Stage #1: 3-(tetrahydropyran-2'-yloxy)propyne With sodium hydride In tetrahydrofuran; dimethyl sulfoxide; mineral oil at 20℃; for 15h; Stage #2: 1-bromo-octane In tetrahydrofuran; dimethyl sulfoxide; mineral oil at 20℃; for 29h; | 82% |
| With n-butyllithium In tetrahydrofuran | |
| Stage #1: 3-(tetrahydropyran-2'-yloxy)propyne With n-butyllithium In tetrahydrofuran at -78℃; for 0.25h; Inert atmosphere; Stage #2: 1-bromo-octane In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide at -78 - 20℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In dichloromethane for 12h; Reflux; | 100% |
| In tetrahydrofuran at 60℃; for 24h; | 95% |
| In ethyl acetate at 20℃; for 24h; | 91% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide In toluene at 120℃; | 100% |
| With sodium hydroxide; triethylamine hydrochloride In water; dimethyl sulfoxide at 80℃; for 8h; | 97.4% |
| With tetrabutylammomium bromide; sodium hydroxide In water; toluene for 24h; Inert atmosphere; Reflux; | 96% |
-
-
6825-20-3
3,6-dibromo-9H-carbazole

-
-
111-83-1
1-bromo-octane

-
-
79554-93-1
3,6-dibromo-9-octyl-9H-carbazole

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide In toluene at 120℃; | 100% |
| With tetrabutylammomium bromide; potassium hydroxide In acetone for 6h; Reflux; | 95% |
| With tetrabutylammomium bromide; potassium hydroxide In water; toluene for 24h; Heating; | 95% |
1-Bromooctane Consensus Reports
1-Bromooctane Specification
The IUPAC name of this chemical is 1-Bromooctane. With the CAS registry number 111-83-1 and EINECS registry number 203-912-9, it is also named as 2-Octyl bromide. In addition, the molecular formula is C8H17Br and the molecular weight is 193.12. It is a kind of clear colourless to yellow-brown liquid and belongs to the classes of Bromine Compounds; Alkyl Bromides; Monofunctional & alpha,omega-Bifunctional Alkanes; Monofunctional Alkanes; Bromine chemicals.
Physical properties about this chemical are: (1)ACD/LogP: 4.87; (2)ACD/LogD (pH 5.5): 4.87; (3)ACD/LogD (pH 7.4): 4.87; (4)ACD/BCF (pH 5.5): 2946.56; (5)ACD/BCF (pH 7.4): 2946.56; (6)ACD/KOC (pH 5.5): 10590.85; (7)ACD/KOC (pH 7.4): 10590.85; (8)#Freely Rotating Bonds: 6; (9)Polar Surface Area: 0 Å2; (10)Index of Refraction: 1.451; (11)Molar Refractivity: 46.84 cm3; (12)Molar Volume: 173.7 cm3; (13)Polarizability: 18.57 ×10-24cm3; (14)Surface Tension: 28.8 dyne/cm; (15)Density: 1.111 g/cm3; (16)Flash Point: 78.3 °C; (17)Enthalpy of Vaporization: 41.92 kJ/mol; (18)Boiling Point: 200.9 °C at 760 mmHg; (19)Vapour Pressure: 0.45 mmHg at 25°C.
Preparation of 1-Bromooctane: it can be prepared by octanol and hydrobromic acid. At first, use sulfuric acid to dissolve the sodium bromide. Then add octanol into the solution and heat it about 7-8 hours with refluxing. After the reaction, seperate the organic phase and wash it with water and concentrated sulfuric acid. You can get the product via drying and distillation. The yield is above 90%.
![]()
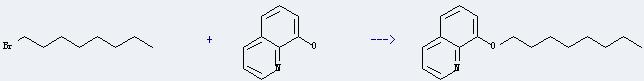
Uses of 1-Bromooctane: it can be used in organic synthesis.And it can react with quinolin-8-ol to get 8-octyloxy-quinoline. This reaction will need reagents KOH and 2 percent Aliquat. The reaction time is 2 hours at reaction temperature of 85 °C. The yield is about 90%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. During using it, you should avoid contact with skin and eyes. And you should not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer).
You can still convert the following datas into molecular structure:
(1)SMILES: BrCCCCCCCC
(2)InChI: InChI=1/C8H17Br/c1-2-3-4-5-6-7-8-9/h2-8H2,1H3
(3)InChIKey: VMKOFRJSULQZRM-UHFFFAOYAC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 8mL/kg (8mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. | |
| rat | LD50 | oral | 4490uL/kg (4.49mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. |
Related Products
- 1-Bromooctane
- 111-84-2
- 1118460-77-7
- 1118-46-3
- 1118-47-4
- 111-85-3
- 111853-51-1
- 1118-61-2
- 111-86-4
- 111865-47-5
- 1118-66-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View