-
Name
1-Methyl-3-phenyl-2-thiourea
- EINECS
- CAS No. 2724-69-8
- Article Data37
- CAS DataBase
- Density 1.194g/cm3
- Solubility
- Melting Point 113°C
- Formula C8H10 N2 S
- Boiling Point 247.4°Cat760mmHg
- Molecular Weight 166.247
- Flash Point 103.4°C
- Transport Information
- Appearance
- Safety Poison by ingestion. Mutation data reported. When heated to decomposition it emits very toxic fumes of SOx and NOx.
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Urea,1-methyl-3-phenyl-2-thio- (6CI,7CI,8CI); 1-Methyl-3-phenyl-2-thiourea;1-Methyl-3-phenylthiocarbamide; 1-Methyl-3-phenylthiourea;1-Phenyl-3-methylthiourea; N-Methyl-N'-phenylthiourea;N-Phenyl-N'-methylthiourea; N1-Methyl-N2-phenylthiourea; NSC 3736
- PSA 56.15000
- LogP 2.06670
Synthetic route

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 3h; | 99% |
| In dichloromethane at 20℃; for 4h; | 98% |
| In tetrahydrofuran at 20℃; for 4h; | 97% |
-
-
593-51-1
methylamine hydrochloride

-
-
103-72-0
phenyl isothiocyanate

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 5h; | 96% |
| With water |

| Conditions | Yield |
|---|---|
| Stage #1: aniline; 3-methyl-1-(methyldithiocarbonyl)imidazolium iodide In ethanol Substitution; Heating; Stage #2: methylamine In ethanol for 2.5h; Substitution; | 94% |


| Conditions | Yield |
|---|---|
| In methanol at 65℃; | 92% |

| Conditions | Yield |
|---|---|
| With capsule network from tris(3-pyridyl)triazine and Co(NCS)2 In Hexadecane for 72h; | 91% |
| In ethanol Heating; | 88% |
| In methanol for 1h; Heating; | 85% |
-
-
1516-85-4, 109314-79-6
tetraphenyloxalic amidine

-
-
556-61-6
methyl thioisocyanate

-
A
-
82627-70-1
1-Methyl-3-phenyl-4,5-diphenylimino-imidazolidin-2-thion

-
B
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| In acetone for 6h; Heating; | A 88% B n/a |

-
-
593-77-1
N-Methylhydroxylamine

-
-
103-72-0
phenyl isothiocyanate

-
A
-
2724-69-8
1-methyl-3-phenylthiourea

-
B
-
102-08-9
N,N-diphenylthiourea

| Conditions | Yield |
|---|---|
| Stage #1: N-Methylhydroxylamine; phenyl isothiocyanate With magnesium oxide In acetone at 20℃; for 0.166667h; Stage #2: for 0.05h; Microwave irradiation; neat (no solvent); | A 76% B 9% |


| Conditions | Yield |
|---|---|
| With trimethylbenzylammonium bromide; triethylamine In water; ethyl acetate for 3h; Heating; | 70% |
-
-
74734-11-5
1H-imidazole-1-carbodithioic acid methyl ester

-
-
62-53-3
aniline

-
-
74-89-5
methylamine

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole-1-carbodithioic acid methyl ester; aniline In ethanol Substitution; Heating; Stage #2: methylamine In ethanol for 6.5h; Substitution; | 65% |


| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 20℃; Cooling with ice; | 61% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Methylhydroxylamine; phenyl isothiocyanate With magnesium oxide In acetone at 20℃; for 0.166667h; Stage #2: for 1h; Microwave irradiation; neat (no solvent); | 22% |
-
-
2438-90-6
N,N'-dimethylthioperoxydicarbamic acid

-
-
62-53-3
aniline

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| In ethanol |
-
-
124-41-4
sodium methylate

-
-
98095-88-6
1-Acetyl-1-methyl-3-methylthiourea

-
A
-
79-20-9
acetic acid methyl ester

-
B
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| In methanol at 25℃; Rate constant; |

-
-
124-41-4
sodium methylate

-
-
98095-87-5
1-Acetyl-1-phenyl-3-methylthiourea

-
A
-
79-20-9
acetic acid methyl ester

-
B
-
98095-88-6
1-Acetyl-1-methyl-3-methylthiourea

-
C
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| In methanol at 25℃; Rate constant; |

-
-
124-41-4
sodium methylate

-
-
98095-86-4
1-Benzoyl-1-phenyl-3-methylthiourea

-
A
-
94398-08-0
1-Benzoyl-1-methyl-3-phenyl-thiourea

-
B
-
2724-69-8
1-methyl-3-phenylthiourea

-
C
-
103-85-5
monophenylthiourea

| Conditions | Yield |
|---|---|
| In methanol at 25℃; Rate constant; var. buffers; |

-
-
124-41-4
sodium methylate

-
-
94398-08-0
1-Benzoyl-1-methyl-3-phenyl-thiourea

-
A
-
2724-69-8
1-methyl-3-phenylthiourea

-
B
-
103-85-5
monophenylthiourea

| Conditions | Yield |
|---|---|
| In methanol at 25℃; Rate constant; |


| Conditions | Yield |
|---|---|
| In dichloromethane Equilibrium constant; Thermodynamic data; |
-
-
67-56-1
methanol

-
-
150715-06-3
5-isopropyl-1-methyl-3-phenyl-2-thiohydantoin

-
A
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With oxygen; sodium methylate for 8h; | A 1 mg B 11 mg C 10 mg |


-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tetrabutylammomium bromide; sodium hydroxide / dichloromethane 2: tetraphosphorus decasulfide / toluene 3: cyclohexa-1,4-diene / acetonitrile / 1 h / UV-irradiation; Inert atmosphere; Photolysis View Scheme |
-
-
54246-62-7
1,4-Dihydro-1-methyl-4-phenyl-5H-tetrazol-5-on

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetraphosphorus decasulfide / toluene 2: cyclohexa-1,4-diene / acetonitrile / 1 h / UV-irradiation; Inert atmosphere; Photolysis View Scheme |

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene In acetonitrile for 1h; UV-irradiation; Inert atmosphere; Photolysis; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: trimethylsilylazide 2: tetrabutylammomium bromide; sodium hydroxide / dichloromethane 3: tetraphosphorus decasulfide / toluene 4: cyclohexa-1,4-diene / acetonitrile / 1 h / UV-irradiation; Inert atmosphere; Photolysis View Scheme |
-
-
556-61-6
methyl thioisocyanate

-
-
62-53-3
aniline

-
-
87-62-7
2,6-dimethylaniline

-
A
-
32767-59-2
1-(2,6-dimethylphenyl)-3-methylthiourea

-
B
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With capsule network from tris(3-pyridyl)triazine and Co(NCS)2 |

-
-
1369761-90-9
[1-bromo-1-(1,1,2,2-tetrafluoroethoxy)methyl]phenylketone

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
1369762-00-4
[3-methyl-4-phenyl-5-(1,1,2,2-tetrafluoroethoxy)-3H-thiazol-2-ylidene]phenylamine

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water at 80℃; for 6h; Hantzsch type cyclization; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| for 0.166667h; microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 1h; Reflux; | 96% |

| Conditions | Yield |
|---|---|
| for 0.166667h; microwave irradiation; | 95% |
-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
16954-69-1
2-methylaminobenzthiazole

| Conditions | Yield |
|---|---|
| With thionyl chloride at 50 - 55℃; for 4h; | 94.8% |
| With bromine; acetic acid at 20℃; for 3h; Hugerschoff reaction; | 83% |
| With bromine In acetic acid for 1h; Ambient temperature; | 75% |
| With sulfuryl dichloride | |
| With lead(II) dihydrogen orthophosphate; H2; buffer solution In methanol for 1.5h; Heating; |
-
-
70-11-1
α-bromoacetophenone

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
97118-81-5
N-(3-methyl-4-phenylthiazol-2(3H)-ylidene)phenylamine

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Heating; | 93.2% |
| With 1,4-diaza-bicyclo[2.2.2]octane In ethanol; water at 20℃; for 0.5h; | 87% |

| Conditions | Yield |
|---|---|
| In acetone | 93% |
-
-
55143-87-8
N-ethylquinoxalinium iodide

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
89607-04-5
(3aS,9aR)-4-Ethyl-3-methyl-1-phenyl-1,3,3a,4,9,9a-hexahydro-imidazo[4,5-b]quinoxaline-2-thione

| Conditions | Yield |
|---|---|
| With diethylamine In ethanol at 20 - 25℃; | 92% |
-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With 1-[4-(diacetoxyiodo)benzyl]-3-methylimidazolium tetrafluoroborate; 1-butyl-3-methylimidazolium Tetrafluoroborate at 25℃; for 1.2h; | 92% |

| Conditions | Yield |
|---|---|
| With dmap; methanesulfonyl chloride; triethylamine In dichloromethane for 0.0833333h; Ambient temperature; | 91% |
| With mercury(II) oxide In dichloromethane; water at 20℃; for 0.5h; | 12% |
| With 2-chloro-1-methyl-pyridinium iodide; triethylamine In N,N-dimethyl-formamide at 20℃; for 1h; |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| for 0.0833333h; microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| for 0.166667h; microwave irradiation; | 90% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
63500-82-3
(Z)-3-methyl-2-(phenylimino)thiazolidine-4-one

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol Reflux; | 90% |
-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
1040550-18-2
2-phenylimino-3-methyl-5-[2-(4-chlorophenyl)-2-oxoethyl]-4-oxo-1,3-thiazolidine

| Conditions | Yield |
|---|---|
| In acetic acid for 1h; Heating; | 89.1% |

| Conditions | Yield |
|---|---|
| In acetone for 4h; Heating; | 89% |
-
-
17648-16-7
bis(dichlorophosphino)methylamine

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With pyridine | A 88% B n/a C n/a |

-
-
35513-39-4
β-(4-bromobenzoyl)acrylic acid

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
1040550-13-7
2-phenylimino-3-methyl-5-[2-(4-bromophenyl)-2-oxoethyl]-4-oxo-1,3-thiazolidine

| Conditions | Yield |
|---|---|
| In acetic acid for 1h; Heating; | 88% |
-
-
19522-26-0
3-benzoyl-2-propenoic acid

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
1040550-08-0
2-phenylimino-3-methyl-5-(2-phenyl-2-oxoethyl)-4-oxo-1,3-thiazolidine

| Conditions | Yield |
|---|---|
| In acetic acid for 1h; Heating; | 87.7% |
-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| With 1-[4-(diacetoxyiodo)benzyl]-3-methylimidazolium tetrafluoroborate; at 25℃; for 2h; | 87% |
-
-
38238-77-6
dimethyl 2-chloroethylene-1,1-dicarboxylate

-
-
2724-69-8
1-methyl-3-phenylthiourea

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 2h; | 87% |
-
-
20595-03-3
N-methylquinoxalinium iodide

-
-
2724-69-8
1-methyl-3-phenylthiourea

-
-
89607-03-4
1,9-dimethyl-3-phenyl-2,3,3a,4,9,9a-hexahydro-1H-imidazo<4,5-b>quinoxaline-2-thione

| Conditions | Yield |
|---|---|
| With diethylamine In ethanol at 20 - 25℃; | 82% |
1-Methyl-3-phenyl-2-thiourea Chemical Properties
Molecule structure of N-Methyl-N'-phenyl thiourea (CAS NO.2724-69-8):
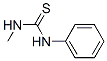
IUPAC Name: 1-Methyl-3-phenylthiourea
Molecular Weight: 166.2434 g/mol
Molecular Formula: C8H10N2S
Density: 1.194 g/cm3
Melting Point: 113 °C
Boiling Point: 247.4 °C at 760 mmHg
Flash Point: 103.4 °C
Index of Refraction: 1.659
Molar Refractivity: 51.37 cm3
Molar Volume: 139.2 cm3
Polarizability: 20.36×10-24 cm3
Surface Tension: 55.8 dyne/cm
Enthalpy of Vaporization: 48.46 kJ/mol
Vapour Pressure: 0.0257 mmHg at 25 °C
XLogP3: 1.7
H-Bond Donor: 2
Rotatable Bond Count: 1
Tautomer Count: 3
Exact Mass: 166.056469
MonoIsotopic Mass: 166.056469
Topological Polar Surface Area: 24.1
Heavy Atom Count: 11
Complexity: 130
Canonical SMILES: CNC(=S)NC1=CC=CC=C1
InChI: InChI=1S/C8H10N2S/c1-9-8(11)10-7-5-3-2-4-6-7/h2-6H,1H3,(H2,9,10,11)
InChIKey: IGEQFPWPMCIYDF-UHFFFAOYSA-N
1-Methyl-3-phenyl-2-thiourea Uses
N-Methyl-N'-phenyl thiourea (CAS NO.2724-69-8) is used to prepare 2-methylamino-benzothiazole which is the intermediate of herbicide methyl benzene thiophene.
1-Methyl-3-phenyl-2-thiourea Production
In the reactor by adding sodium carbonate solution, the carbon disulfide solution of aniline added to the reactor, the reaction temperature was controlled at 40 ~ 50 °C, generating two thio aniline sodium (PhNHCS2Na), and then join the methylamine solution and keep stirring obtained crystallization, filtration, washing derived product.
1-Methyl-3-phenyl-2-thiourea Toxicity Data With Reference
| 1. | dlt-mus-ipr 50 mg/kg | MGGEAE Molecular and General Genetics. 117 (1972),197. | ||
| 2. | orl-rat LDLo:50 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 93 (1948),287. |
1-Methyl-3-phenyl-2-thiourea Consensus Reports
Reported in EPA TSCA Inventory.
1-Methyl-3-phenyl-2-thiourea Safety Profile
Poison by ingestion. Mutation data reported. When heated to decomposition it emits very toxic fumes of SOx and NOx.
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
1-Methyl-3-phenyl-2-thiourea Specification
N-Methyl-N'-phenyl thiourea (CAS NO.2724-69-8) is also called 1-Methyl-3-phenylthiocarbamide ; 1-Methyl-3-phenylthiourea ; 1-Phenyl-3-methylthiourea ; 3-12-00-00853 (Beilstein Handbook Reference) ; BRN 0638006 ; N-Methyl-N'-phenylthiourea ; NSC 3736 ; Thiourea, N-methyl-N'-phenyl- ; Urea, 1-methyl-3-phenyl-2-thio- . N-Methyl-N'-phenyl thiourea (CAS NO.2724-69-8) is lamellae. It can soluble in ethanol and ether and also can dissolve in water.
Related Products
- 1-Methyl-3-phenyl-2-thiourea
- 27247-41-2
- 27247-96-7
- 272-49-1
- 272-50-4
- 27251-84-9
- 2725-22-6
- 272-52-6
- 27253-29-8
- 27253-31-2
- 27253-58-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View